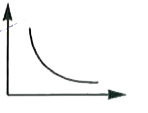
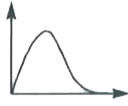
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS
AAKASH SERIES|Exercise EXERCISE -IB|44 VideosATOMS
AAKASH SERIES|Exercise EXERCISE -II|35 VideosATOMS
AAKASH SERIES|Exercise PRACTICE EXERCISE|21 VideosAPPENDICES (REVISION EXERCISE)
AAKASH SERIES|Exercise LAW OF MOTION|128 VideosCAPACITORS
AAKASH SERIES|Exercise PRACTICE SHEET (ADVANCED) (Integer Type Questions)|2 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ATOMS-EXERCISE -IA
- In the geiger -marsden scattering experiment the number of scattered d...
Text Solution
|
- The volume occupied by an atom is greater than the volume of the nucle...
Text Solution
|
- The graph of the total number of alpha-particles scattered at differen...
Text Solution
|
- According to Bohr's principle , the relation between principle quantum...
Text Solution
|
- Find the longest and shortest wavelengths in the Lyman series for hy...
Text Solution
|
- In a p-n junction, depletion region contains
Text Solution
|
- An electron in Bohr's hydrogen atom has an energy of -3.4eV. The angul...
Text Solution
|
- If radius of second stationary orbit (in Bohr's atom) is R then radius...
Text Solution
|
- Find angular momentum of an electron when it is in the second Bohr orb...
Text Solution
|
- the energy required to excite an electron in hydrogen atom to its firs...
Text Solution
|
- When electron jumps from n = 4 to n = 1 orbit, we get
Text Solution
|
- When an electrons jumps from higher orbit to the second orbit in He^+ ...
Text Solution
|
- The total energy of electron in the ground state of hydrogen atom is -...
Text Solution
|
- The diagram shows the energy levels for an electron in a certain atom....
Text Solution
|
- The wavelengths involved in the spectrum of deuterium (.(1)^(2)D) are...
Text Solution
|
- When a hydorgen atom is excited from ground state to first excited sta...
Text Solution
|
- Which of the following is not correct about bohr model of the hydrogen...
Text Solution
|
- In the Bohr's model of a hydrogen atom, the centripetal force is furni...
Text Solution
|
- The Bohr model of atoms
Text Solution
|
- Bohr's model of hydrogen atom.
Text Solution
|