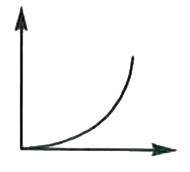
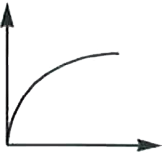
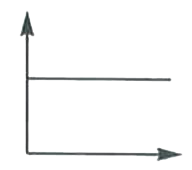
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS
AAKASH SERIES|Exercise EXERCISE -IB|44 VideosATOMS
AAKASH SERIES|Exercise EXERCISE -II|35 VideosATOMS
AAKASH SERIES|Exercise PRACTICE EXERCISE|21 VideosAPPENDICES (REVISION EXERCISE)
AAKASH SERIES|Exercise LAW OF MOTION|128 VideosCAPACITORS
AAKASH SERIES|Exercise PRACTICE SHEET (ADVANCED) (Integer Type Questions)|2 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ATOMS-EXERCISE -IA
- Which of the following parameters are the same for all hydrogen like a...
Text Solution
|
- The angular momentum of an electron in a hydrogen atom is proportional...
Text Solution
|
- The electron in a hydrogen atom makes a transition from an excited sta...
Text Solution
|
- In a hydrogen atom, the radius of n^(th) bohr orbit is rn. The graph b...
Text Solution
|
- How many waves will be made by an electron in the hydrogen atom if it ...
Text Solution
|
- An electron in the ground state of hydrogen atom is revolving in antic...
Text Solution
|
- when an electron falls from a higher energy to a lower energy level th...
Text Solution
|
- The de- broglie wavelength of an electron in the first bohr orbit is
Text Solution
|
- Bohr's basic idea of discrete energy levels in atoms and the process o...
Text Solution
|
- From quantisation of angular momentum one gets for hydrogen atom, the ...
Text Solution
|
- the excitation energy of lyman last lines is
Text Solution
|
- The change in orbital angular momentum corresponding to an electron tr...
Text Solution
|
- The minimum magnetic dipole moment of electron in hydrogen atom is
Text Solution
|
- Total energy of the electron in hydrogen atom above 0 eV leads to
Text Solution
|
- An ionized H-molecule consists of an electron and two protons. The pro...
Text Solution
|
- Consider aiming a beam of free electons towards free protons. When the...
Text Solution
|
- The simple Bohr model is not applicable to He^(4) atom because
Text Solution
|
- When a hydrogen atom is raised from the ground state to an excited sta...
Text Solution
|
- The simple Bohr model is not applicable to He^(4) atom because
Text Solution
|
- Check the corretness of the following statement about the Bohr model o...
Text Solution
|