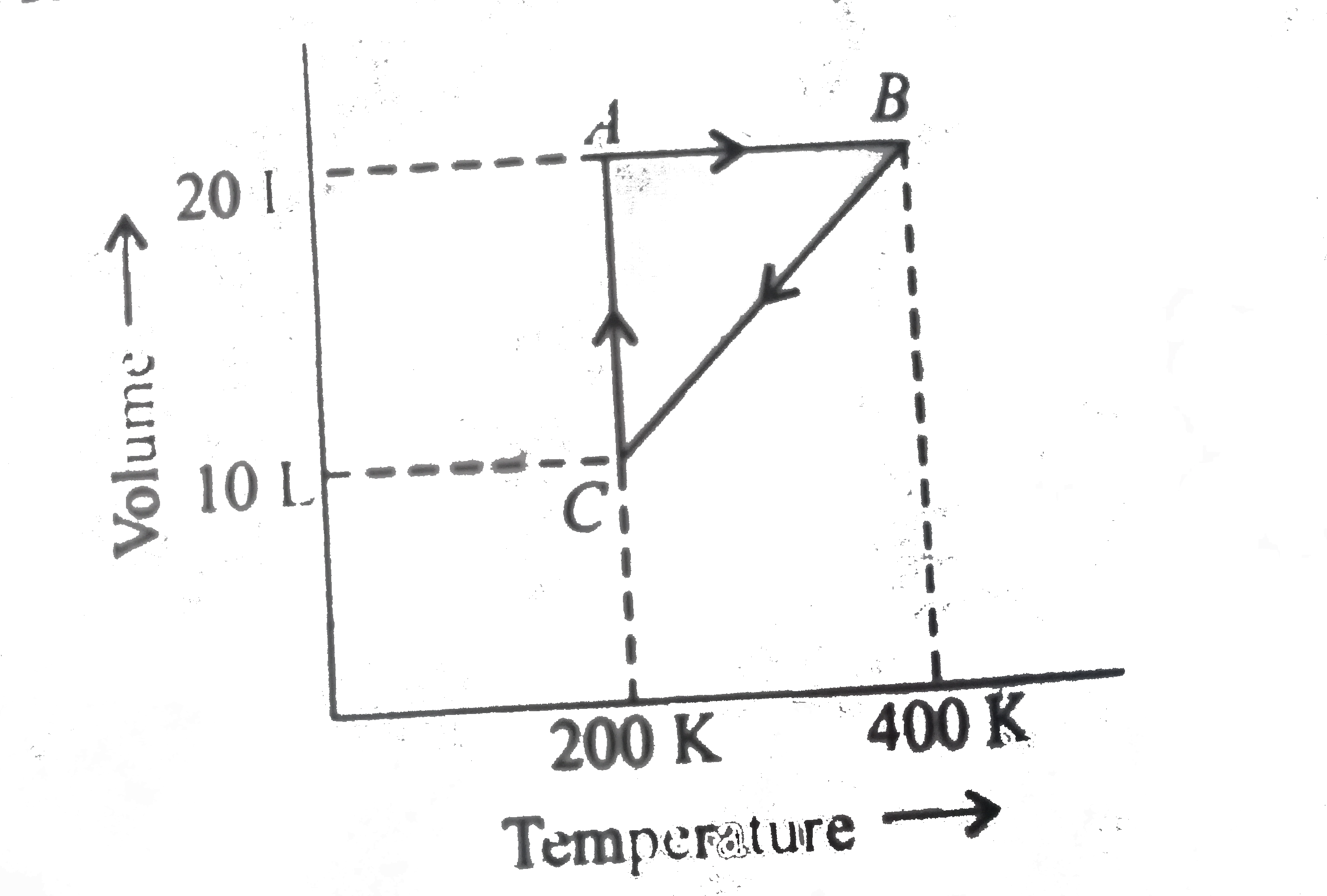
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
WAVES OPTICS
AAKASH SERIES|Exercise EXERCISE -IA (MORE THAN ONE OPTION)|14 VideosWAVES OPTICS
AAKASH SERIES|Exercise EXERCISE -IA (MATCHING TYPE QUESTIONS)|6 VideosWAVES OPTICS
AAKASH SERIES|Exercise EXERCISE -IA (POLARASATION)|29 VideosWAVES
AAKASH SERIES|Exercise EXERCISE-III (Doppler effect :)|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-WAVES OPTICS-EXERCISE -IA (SATEMENT TYPE QUESTIONS)
- Consider the following statement A and B and identify the correct answ...
Text Solution
|
- Instead of using two slits as in young.s experiment, if we use two sep...
Text Solution
|
- Consider the following statement A and B and identify the correct answ...
Text Solution
|
- Consider the following statement A and B and identify the correct answ...
Text Solution
|
- In the following diagram label A and B
Text Solution
|
- Consider the following statement A and B and identify the correct answ...
Text Solution
|
- Consider the following statement A and B and identify the correct answ...
Text Solution
|
- Consider the following statement A and B and identify the correct answ...
Text Solution
|
- In the following diagram label A and B
Text Solution
|
- The pressures at A and B in the atmosphere are, respectively,
Text Solution
|
- In a reaction, DeltaH and DeltaS both are more than zero. In which of ...
Text Solution
|
- A man in a lift ascending with an upward acceleration a throws a ball ...
Text Solution
|