A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-DUAL NATURE OF RADIATION AND MATTER-PRACTICE EXERCISEX
- If the work function for a certain metal is 3.2 xx 10^(-19) J and it ...
Text Solution
|
- Work function of a metal is 3.0 eV. It is illuminated by a light of wa...
Text Solution
|
- A metal sheet of silver is exposed to ultraviolet radiation of wavelen...
Text Solution
|
- The threshold wavelength for emission of photoelectrons from a metal s...
Text Solution
|
- What will be the minimum frequency of light source to get photocurrent...
Text Solution
|
- A stationary shell of mass M explodes in to two parts and their masses...
Text Solution
|
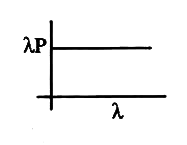
- The graph between deBroglie wavelength (lamda) and (lamdap) is (p is t...
Text Solution
|
- The de Broglie wavelength of an electron is 1A^(@). In what potential ...
Text Solution
|
- If the velocity of the particle reduced to one third, then the percent...
Text Solution
|
- The ratio of the deBroglie wavelengths of proton, deuteron and alpha p...
Text Solution
|
- Find the momentum of an electron having wavelength 2A^(0)(h=6.62xx10^(...
Text Solution
|
- Calculate the wavelength (in nanometer) associated with a proton movin...
Text Solution
|
- <img src="https://d10lpgp6xz60nq.cloudfront.net/physicsimages/BMSDPP01...
Text Solution
|
- A block of mass m is at rest relative to the stationary wedge of mass ...
Text Solution
|
- STATEMENT -1 : Work function of a metal depends on ionisation energy....
Text Solution
|
- With a d.c power supply giving 10mA at a p.d of 50 kV. The power of th...
Text Solution
|
- Iron (z = 26) emits k(alpha) line of wavelength 1.96A^(@). For an elem...
Text Solution
|
- Obtain a relation between the frequencies of K(alpha), K(beta) and L(a...
Text Solution
|
- The energy of a photon is equal to the kinetic energy of a proton. T...
Text Solution
|
- The radiation corresponding to 3 rarr 2 transition of hydrogen atom fa...
Text Solution
|