A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ATOMS-PRACTICE EXERCISE
- Hydrogen atom emits blue light when it jumps from n=4 energy level to ...
Text Solution
|
- The separation energy of the electron presetn in the shell n =3 is 1.5...
Text Solution
|
- Radius of first orbit of hydrogen is 0.53 Å. The radius in fourth orbi...
Text Solution
|
- The electrons in hydrogen atoms are raised from ground state to third ...
Text Solution
|
- For H^(+) and Na^(+) the values of lambda^(@) are 349.8 and 50.11 resp...
Text Solution
|
- Calculated the energy required to excite one litre of hydrogen gas at ...
Text Solution
|
- The ratio of the energies of the hydrogen atom in its first excited st...
Text Solution
|
- Find the ratio of wavelengths of first line of Lyman series and second...
Text Solution
|
- Calculated the energy required to excite one litre of hydrogen gas at ...
Text Solution
|
- In Bohr's model of the hydrogen atom, the ratio between the period of ...
Text Solution
|
- The radius of the first orbit of hydrogen in 0.528 overset(0)A. The ra...
Text Solution
|
- The ovary is half inferior in flowers of
Text Solution
|
- The circumference of first orbit of hydrogen atom is .S.. Then the Bro...
Text Solution
|
- In hydrogen spectrum L(alpha) line arises due to transition of electro...
Text Solution
|
- If a hydrogen atom emit a photon of energy 12.1 eV , its orbital angul...
Text Solution
|
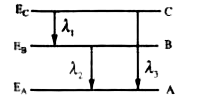
- Energy levels A,B,C of an atom are shown below If E(A)ltE(B)ltE(C...
Text Solution
|
- An electron, in a hydrogen like atom , is in excited state. It has a t...
Text Solution
|
- What is the energy of state in a triply ionized beryllium (Be^(+++)) w...
Text Solution
|
- A stationary hydrogen atom emits photon corresponding to the first lin...
Text Solution
|
- The radius of the first orbit of hydrogen is rH and the energy in th...
Text Solution
|