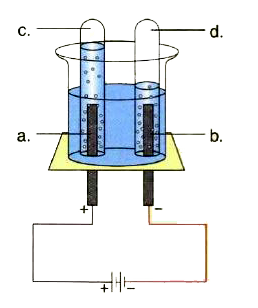
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CAPACITORS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE - II) (LEVEL - I (ADVANCED)) (Integer Type Questions )|1 VideosCAPACITORS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE - III) (LEVEL - I (MAIN)) (Straight Objective Type Questions )|3 VideosCAPACITORS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE - II) (LEVEL - I (ADVANCED)) (More than one correct answer Type Questions )|1 VideosATOMS
AAKASH SERIES|Exercise PRACTICE EXERCISE|21 VideosCOMMUNICATION SYSTEM
AAKASH SERIES|Exercise LECTURE SHEET (LEVEL - I) (MAIN) (EXERCISE - IV) (Straight Objective & More than one Correct Answer Type Questions)|20 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CAPACITORS -LECTURE SHEET (EXERCISE - II) (LEVEL - I (ADVANCED)) (Linked ComprehensionType Questions )
- The given circuit shows an arrangement of four capacitors. A potential...
Text Solution
|
- The given circuit shows an arrangement of four capacitors. A potential...
Text Solution
|
- The given circuit shows an arrangement of four capacitors. A potential...
Text Solution
|
- The figure shows a diagonal symetric arrangement of capacitors and a b...
Text Solution
|
- Shows a diagonal symmetric arrangement of capacitors and a battery. ...
Text Solution
|
- Shows a diagonal symmetric arrangement of capacitors and a battery. ...
Text Solution
|
- Name the process shown in the figure.
Text Solution
|