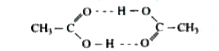Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Acetic acid shows a molecular mass of 120 when dissolved in benzene. T...
Text Solution
|
- Acetic acid dissolved in benzene shows a molecular mass of:
Text Solution
|
- The molecular mass of acetic acid dissolved in water is 60 and when di...
Text Solution
|
- Acetic acid dissolved in benzene shows a molecular mass of g...
Text Solution
|
- Acetic acid has molecular weight of 120 in benzene solution. This is d...
Text Solution
|
- Acetic acid dissolved in benzene shows a molecular mass of:
Text Solution
|
- Acetic acid undergoes dimerisation , when dissolved in benzene Mo...
Text Solution
|
- Acetic acid undergoes dimerisation, when dissolved in benzene Mol...
Text Solution
|
- Acetic acid shows a molecular mass of 120 when dissolved in benzene. T...
Text Solution
|