A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
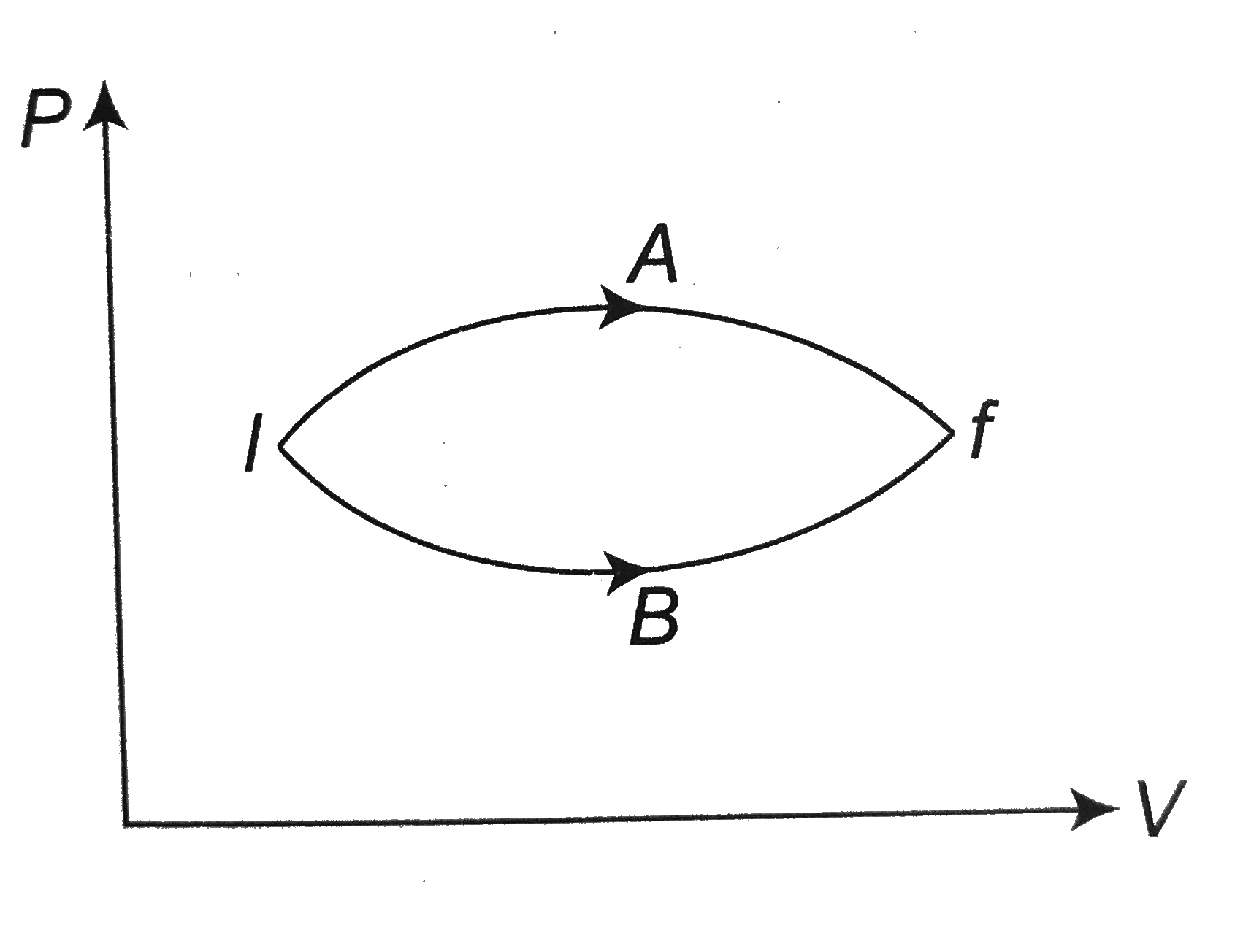
- In the figure given two processes A and B are shown by which a thermod...
Text Solution
|
- shows two processes A and B on a system.Let DeltaQ(1) and DeltaQ(2) b...
Text Solution
|
- consider two processes on a system on a system as shown in the volum...
Text Solution
|
- A system goes from A and B via two processes. I and II as shown in fig...
Text Solution
|
- In the figure given two processes A and B are shown by which a thermod...
Text Solution
|
- Consider the two process on a system as shown in figure. The volumes i...
Text Solution
|
- चित्र में गैस के सेम्पल के लिए दो प्रक्रम a व b दिये गये हैं। यदि Delt...
Text Solution
|
- Figure shows two prpcesses A and B on a system. Let DeltaQ(1) and Delt...
Text Solution
|
- Consider two processes on a system as shown in figure. The volumes in ...
Text Solution
|