A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
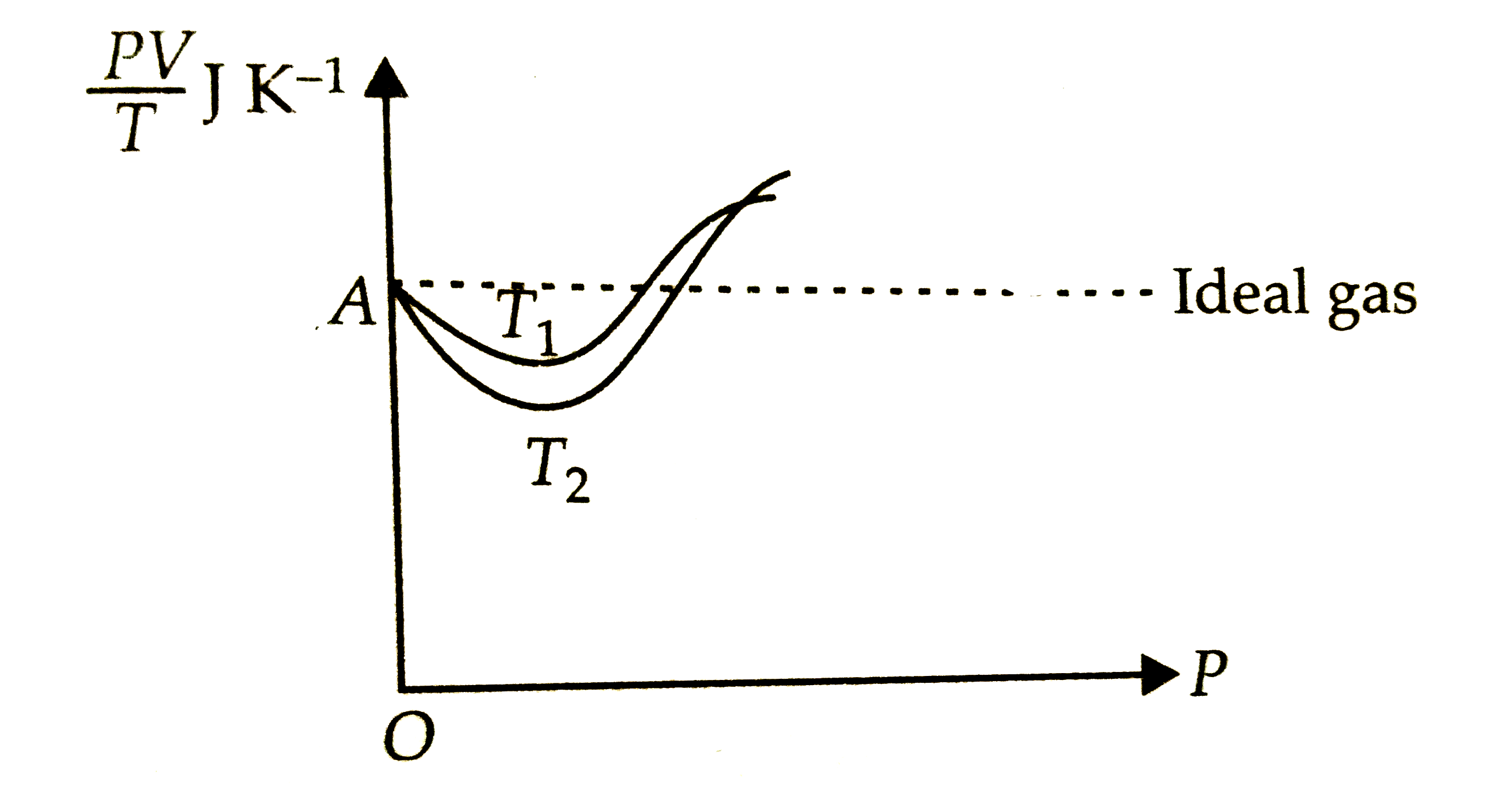
- Given is the graph between (PV)/(T) and P for 1 g of oxygen gas at two...
Text Solution
|
- Given is the graph between (PV)/T and P for 1 gm of oxygen gas at two ...
Text Solution
|
- The product of PV is potted aginst P at two temperatures T(1) and T(2)...
Text Solution
|
- The voltage V and current I graph for a conductor at two different tem...
Text Solution
|
- The product of PV is plotted against P at two temperatures T(1) and T(...
Text Solution
|
- The voltage V and current I v graphs for a conductor at two different ...
Text Solution
|
- चित्र में विभवांतर V और धारा I के बीच किसी चालक के दो ताप T(1)और ...
Text Solution
|
- The density (rho) versus pressure (P) graphs of a given mass of an ide...
Text Solution
|
- At two different temperature T(1) and T(2), (T(2) gt T(1)) , draw two ...
Text Solution
|