Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- A metre long narrow bore held horizontally (and closed at one end) con...
Text Solution
|
- A column of mercury of 10cm length is contained in the middle of a nar...
Text Solution
|
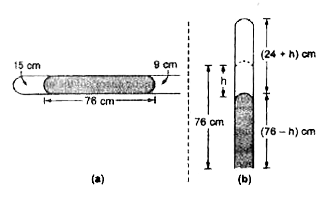
- A narrow uniform glass tube contains air enclosed by 15 cm long thread...
Text Solution
|
- A meter long narrow bore held horizontally (and closed at one end) con...
Text Solution
|
- A metre long narrow bore held horizontally (and close at one end) cont...
Text Solution
|
- A 10.0 cm column of air is trapped by a column of Hg 4.00 cm long in c...
Text Solution
|
- A uniform tube closed at one end contains some air confined by a mercu...
Text Solution
|
- A metre long narrow bore held horizontally (and closed at one end) con...
Text Solution
|
- A metre long narrow bore held horizontally (and closed at one end) con...
Text Solution
|