Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
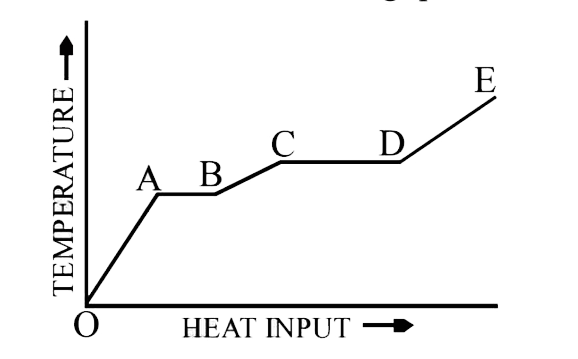
- A solid material is supplied with heat at a constant rate. The tempera...
Text Solution
|
- A solid material is supplied with heat at a constant rate. The tempera...
Text Solution
|
- A solid material is supplied heat at a constant rate. The temperature...
Text Solution
|
- A solid material is supplied with heat at a constant rate. The tempera...
Text Solution
|
- When we raise the temperature of a body, the molecules and atoms move ...
Text Solution
|
- A source of heat supplies heat at constant rate to a solid cube. The v...
Text Solution
|
- The figure shows the effect of light on the rate of photosynthesis. Ba...
Text Solution
|
- A source of heat supplies heat at a constant rate to solid cube. The ...
Text Solution
|
- A source of heat supplies heat at a constant rate to solid cube. The v...
Text Solution
|