A group between x/m and the presure P of the gas at a constant temperature is called adsorption siotherm. Where x is the no. of moles of the adsorbate and m is the mass of the adsorbent. adsoption isotherms of different shaopes have been experimentally observed .. According to frundlich adosroption isthem,
`x//m = KP^(1//n)`
where K and N are constant paraments depending upon the nature of the solid and gas
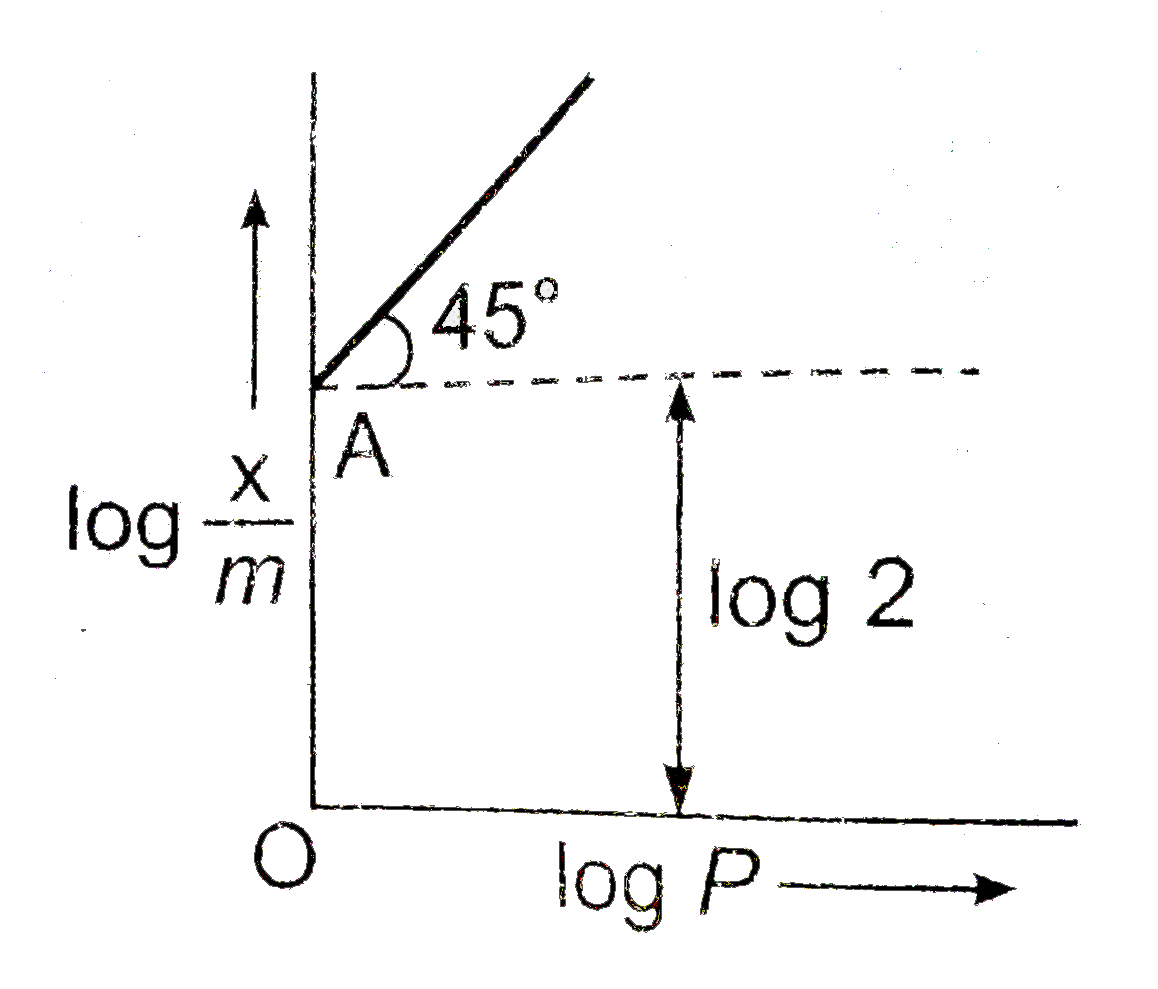
graph between log `(x/m)` and log P is a straight line a t angle `45^(@)` with intercept OA as shown .
hence,` (x/m)` at a pressure of 2 atm is :