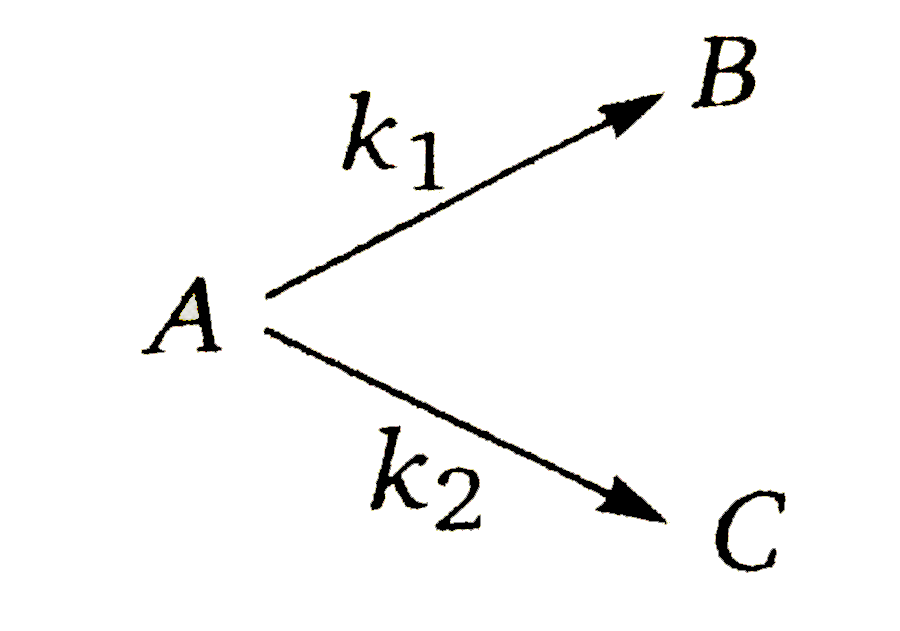
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETIC & NUCLEAR CHEMISTRY
NARENDRA AWASTHI|Exercise Level 2 (Q.33 To Q.35)|2 VideosCHEMICAL KINETIC & NUCLEAR CHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Passage|1 VideosCHEMICAL KINETIC & NUCLEAR CHEMISTRY
NARENDRA AWASTHI|Exercise Level 2|2 VideosSURFACE CHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Assertion - Reason Type Questions|1 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-CHEMICAL KINETIC & NUCLEAR CHEMISTRY-Level 2 (Q.3 To Q.32)
- The reaction A(g)toB(g)+2C(g) is a first order reaction with rate cons...
Text Solution
|
- A(aq)rarrB(aq)+C(aq) is first order reaction. "Time"" "t" "oo "...
Text Solution
|
- The gaseous decomposition reaction, A(g)rarr2B(g)+C(g) is observed to ...
Text Solution
|
- The reaction ,Sucroseoverset(H^(+))rarr Glucose + Fructose, take place...
Text Solution
|
- A gaseous compound A reacts by three independent first order process (...
Text Solution
|
- A compound A dissociates by two parallel first order paths at certain ...
Text Solution
|
- For given hypothetical elementary parallel reaction, where k1/k2 = 1/2...
Text Solution
|
- The reaction cis-Xunderset(k(b))overset(k(f))ltimplies trans-X is fir...
Text Solution
|
- Consider the reaction. The rate constant for two parallel reacti...
Text Solution
|
- A reaction takes place in various steps. The rate constatn for first, ...
Text Solution
|
- For reaction ArarrB, the rate constant k(1)=A(1)e^(-Ea(1//(RT))) and f...
Text Solution
|
- For first order parallel reactions k(1) and k(2) are 4and 2min(-1) re...
Text Solution
|
- In the series reaction Aoverset(K(2))(rarr)Boverset(K(2))(rarr)Cover...
Text Solution
|
- The mechanism of esterification in presence of acid catalyst (H(2)SO(...
Text Solution
|
- For the first order reaction A rarr B +C , carried out at 27^(@) C . I...
Text Solution
|
- Upon irradiating californium with neutrons, a scientist discovered a ...
Text Solution
|
- The average (mean) life of a radio nuclide which decays by parallel pa...
Text Solution
|
- The radioactive decay .(83)^(211)Birarr(81)^(207)Ti , takes place in ...
Text Solution
|
- A fresh radioactive mixture contians short lived sppecies A and B. Bot...
Text Solution
|
- In order to determine the volume of blood in an animal, a 1.0mL sample...
Text Solution
|