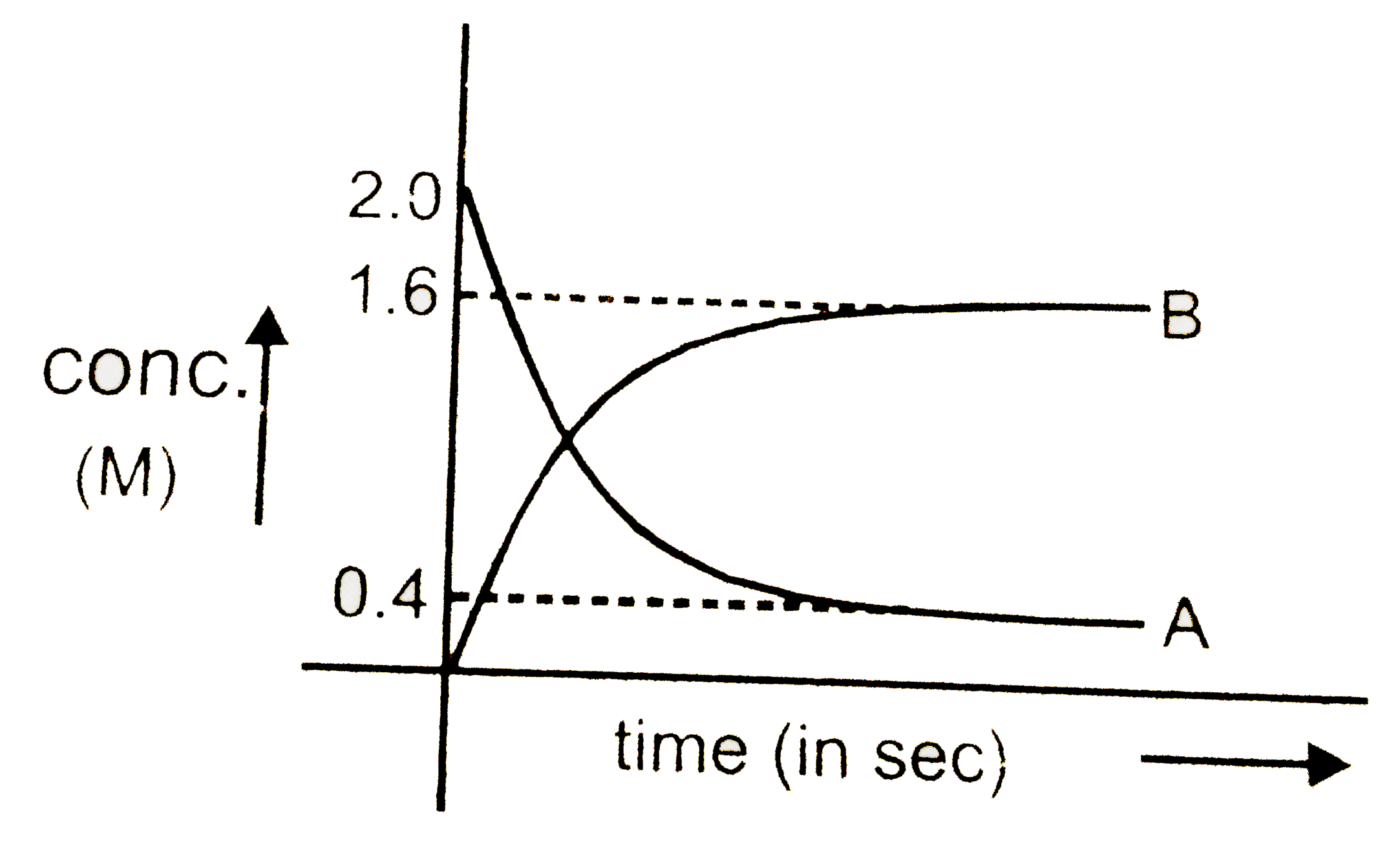
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETIC & NUCLEAR CHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Assertion - Reason Type Questions|3 VideosCHEMICAL KINETIC & NUCLEAR CHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|3 VideosCHEMICAL KINETIC & NUCLEAR CHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Passage|1 VideosSURFACE CHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Assertion - Reason Type Questions|1 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-CHEMICAL KINETIC & NUCLEAR CHEMISTRY-Level 3 - One Or More Answers Are Correct
- Select the correct statement(s) :
Text Solution
|
- Consider a reaction A+B rarr C , in which boht reactants are in the sa...
Text Solution
|
- In the following gaseous phase first order reaction A(g) rarr 2B(g)+...
Text Solution
|
- Indentify the true statement(s)
Text Solution
|
- For the reaction Aunderset(k(2)"sec"^(-1))overset(K(1)"sec"^(-1))hArr ...
Text Solution
|
- Select the correct statement(s) :
Text Solution
|
- Select the correct statement(s) :
Text Solution
|
- Select the correct statement(s) :
Text Solution
|
- In electron capture (radioactive process):
Text Solution
|
- Select the correct statement(s) for positron emission by unstable nucl...
Text Solution
|
- Select the correct statement(s)
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|