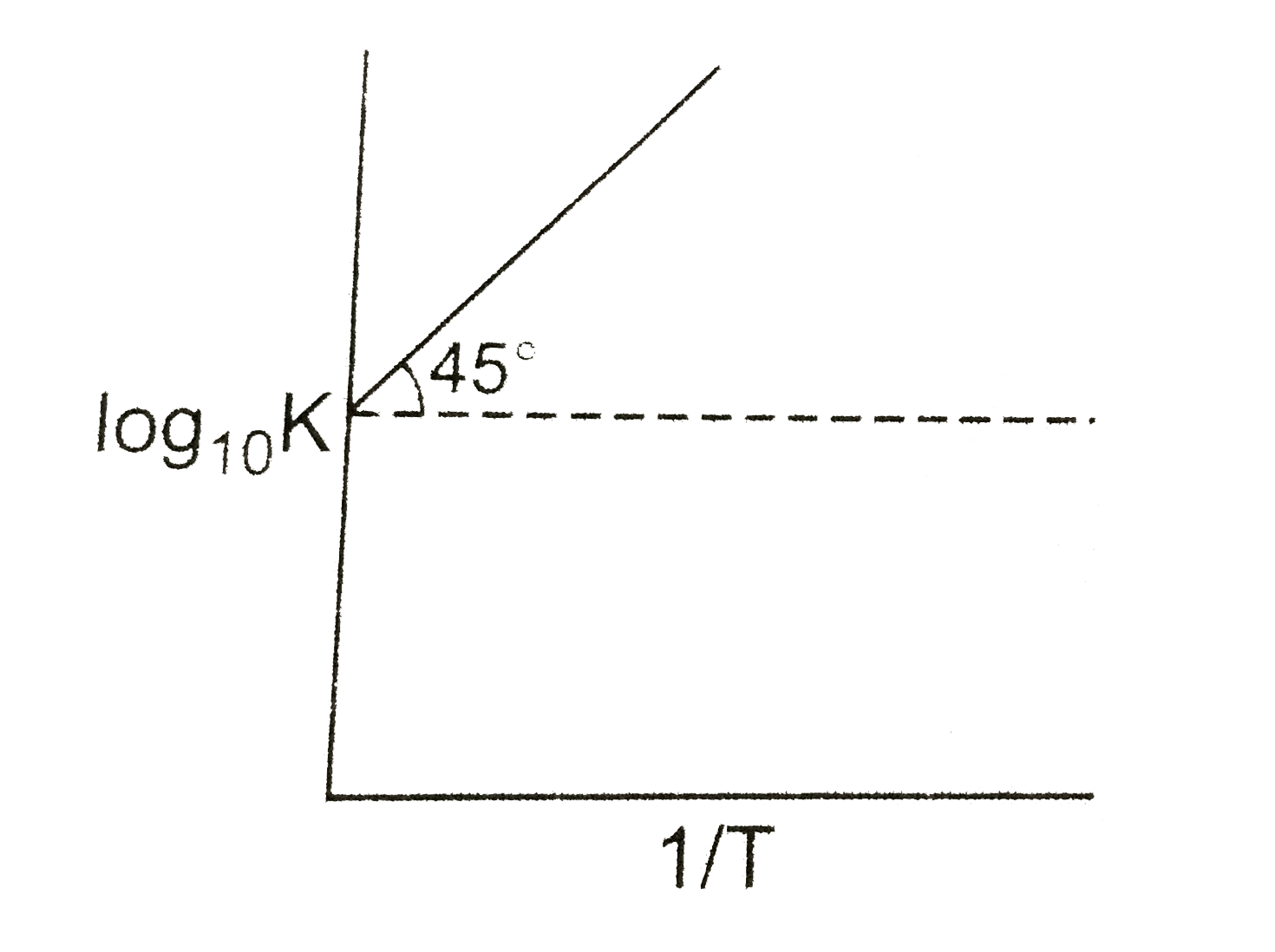
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Variation of equilibrium constan K with temperature is given by van't ...
Text Solution
|
- The temperature dependence of equilibrium constant of a reaction is gi...
Text Solution
|
- Standard Gibbs energy of reaction (Delta(r)G^(@)) at a certain tempera...
Text Solution
|
- Standard Gibbs energy of reaction (Delta(r)G^(@)) at a certain tempera...
Text Solution
|
- Standard Gibbs energy of reaction (Delta(r)G^(@)) at a certain tempera...
Text Solution
|
- Standard Gibbs energy of reaction (Delta(r)G^(@)) at a certain tempera...
Text Solution
|
- Variation of equilibrium constan K with temperature is given by van't ...
Text Solution
|
- Variation of equilibrium constan K with temperature is given by van't ...
Text Solution
|
- Standard Gibb's energy of reaction (Delta(r )G^(@)) at a certain temp...
Text Solution
|