Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER 12-SECTION - B
- Deduce (a) Boyle's law and (b) Charles law from Kinetic gas equation.
Text Solution
|
- Balance the following redox reactions by ion-electron method : MnO...
Text Solution
|
- Which buffer solution has maximum pH?
Text Solution
|
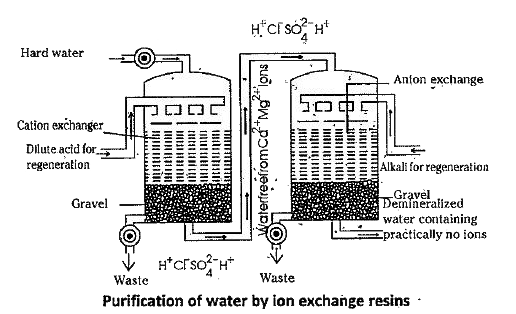
- Discuss the principle and the method of softening of hard water by syn...
Text Solution
|
- What is Plaster of Paris? Write a short note on it.
Text Solution
|
- Explain borax bead test with a suitable example
Text Solution
|
- Explain the following : b) Thin layer chromatography
Text Solution
|
- In paper chromatography
Text Solution
|
- What is dehydrologenation? Write the equation for the formation of alk...
Text Solution
|