Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-Unit test - Properties of bulk matter-Example
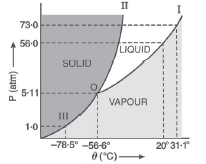
- Answer the following questions based on the p-T phase diagram of carbo...
Text Solution
|
- Answer the following questions based on the p-T phase diagram of carbo...
Text Solution
|
- Answer the following questions based on the p-T phase diagram of carbo...
Text Solution
|
- Answer the following questions based on the P-T phase diagram of CO2 ...
Text Solution
|
- Answer the following questions based on the P-T phase diagram of CO2 ...
Text Solution
|
- Answer the following questions based on the P-T phase diagram of CO2 ...
Text Solution
|
- A child running a temperature of 101^@F is given an antipyrin (i.e. a ...
Text Solution
|
- A thermocole box is a cheap and effieicnet method for storing small qu...
Text Solution
|
- A brass boiler has a base area of 0.15 m^2 and thickness 1.0 cm. It bo...
Text Solution
|
- Explain why: A body with large reflecting is a poor emitter.
Text Solution
|
- Explain why: A brass tumbler feels much colder than a wooden tray on...
Text Solution
|
- Explain why: An optical pyrometer (for measuring high temperature) c...
Text Solution
|
- Explain why: The earth without its atmosphere would be in hospitably...
Text Solution
|
- Explain why: Heating systems based on circulation of steam are more ...
Text Solution
|
- A body cools from 80^@C to 50^@C in 5 minuts. Calculate the time it ta...
Text Solution
|
- A wire elongates by l mm when a load W is hanged from it. If the wire ...
Text Solution
|
- Two wires are made of the same material and have the same volume. Howe...
Text Solution
|
- If S is stress and Y is Young's modulus of material of a wire, find th...
Text Solution
|
- A wire fixed at the upper end stretches by length l by applying a forc...
Text Solution
|
- A wire suspended vertically from one of its ends is stretched by attac...
Text Solution
|