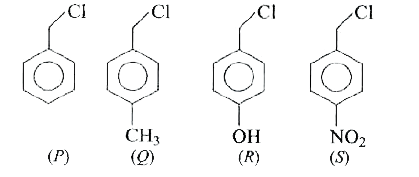
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Rate of SN^1
Text Solution
|
- Which one hydrolyses at a faster rate by SN^1 mechanism ? .
Text Solution
|
- Rate of ethanolysis of 1^@ halide by SN^1 machanism is fast. Carocatio...
Text Solution
|
- Which compound undergoes nucleophilic substitionSN^1 with NaCN at th...
Text Solution
|
- Give the order of SN^(-1) of SN^(-1) and SN^(2) displacement of haloge...
Text Solution
|
- SN^(1) and SN^(2) mechanisms
Text Solution
|
- Relative rate of SN^(1) reaction for t-butyl bromide?
Text Solution
|
- Relative rate of methyl bromide in SN^(2) reaction is
Text Solution
|
- The rate of SN^(1) reaction is fastest with
Text Solution
|