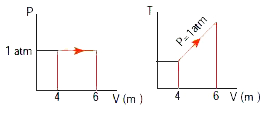Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT TAMIL-HEAT AND THERMODYNAMICS-EVALUATION (Numerical Problems)
- Calculate the number of moles of air is in the inflated balloon at roo...
Text Solution
|
- In the planet Mars, the average temperature is around -53^(@)C and atm...
Text Solution
|
- An insulated container of gas has two chambers separated by an insulat...
Text Solution
|
- The temperature of a uniform rod of length L having a coefficnet of li...
Text Solution
|
- A man starts bicycling in the morning at a temperature around 25^(@)C,...
Text Solution
|
- Normal human body of the temperature is 98.6^(@)F. During high fever i...
Text Solution
|
- In a petrol engine, (internal combustion engine) air at atmospheric pr...
Text Solution
|
- Consider the following cyclic process consist of isotherm, isochoric a...
Text Solution
|
- An ideal gas is taken in a cyclic process as shown in the figure. Calc...
Text Solution
|
- For a given ideal gas 6xx10^(5)J heat energy is supplied and the volum...
Text Solution
|
- Suppose a person wants to increase the efficiency of the reversible he...
Text Solution
|
- A Carnot engine whose efficiency is 45% takes heat from a source maint...
Text Solution
|
- An ideal refrigerator keeps its content at 0^@C while the room tempera...
Text Solution
|