Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-MODEL PAPER 11-SECTION - B
- State and explain Graham's law of Diffusion.
Text Solution
|
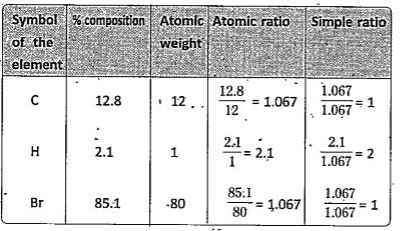
- A carbon compound contains 12.8% Carbon, 2.1% Hydrogen, 85.1% Bromine....
Text Solution
|
- Discuss the application of LE Chatellier's principle for the industria...
Text Solution
|
- The reactants in the industrial method of preparation of diborane are
Text Solution
|
- Write a few lines about cement.
Text Solution
|
- Explain the structure of diborane.
Text Solution
|
- What is substitution reaction? Explain any two substitution reactions...
Text Solution
|
- Explain cryatallization and sublimation phenomena whilch are used in t...
Text Solution
|