Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA TPC JEE MAIN TEST 40-CHEMISTRY
- The ratio of the rate of a reaction at 40^(@)C to the rate of a reacti...
Text Solution
|
- What will be the equivalent conductance of MgSO(4) at infinite dilutio...
Text Solution
|
- What is the minimum pH required to prevent the precipitation of ZnS in...
Text Solution
|
- Which of the following expression is is correct in case of a CsCl unit...
Text Solution
|
- A solution of 0.5 g of a solute ( molar mass =150gmol^(-1)) in 50 g of...
Text Solution
|
- A liquid has 35 drops in 2mL. The density of the liquid is 1.2 g/ml. H...
Text Solution
|
- Vander waal's equation for 1 -mole He gas at high pressure can be writ...
Text Solution
|
- The energy of an electron in the first orbit of the hydrogen atom is ...
Text Solution
|
- At high pressure, Langmuir adsorption isotherm takes the form:
Text Solution
|
- A person requires 2870 kcal of energy, to lead a normal daily life. If...
Text Solution
|
- Find out the total number of diamagnetic species is : K(2)O(2),[Ni(N...
Text Solution
|
- The total Ione pairs of electrons in XeOF(4) is
Text Solution
|
- How many electrons are present in the catalyst of the lead chamber pro...
Text Solution
|
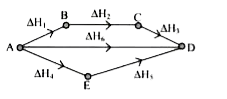
- Consider the following process for the conversion of A to D. How m...
Text Solution
|
- Insulin is used to treat a number of diseases including diabetes and i...
Text Solution
|
- One mole each of calcium acetate and calcium propionate undergoes dry ...
Text Solution
|
- A saturated alkyl halide (C(3)H(7)X), when heated with dry silver oxid...
Text Solution
|
- a. number of structural isomeeric products. b. Number of meso f...
Text Solution
|
- How many P-O-P linkage (s) are present in P(4)O(12)^(-4)?
Text Solution
|
- 60 ml NaOH solution is required for complete neutralisation of 0.98 gm...
Text Solution
|