Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA TPC JEE MAIN TEST 57-CHEMISTRY
- When isobutane is treated with Br2 in sunlight, then major product is ...
Text Solution
|
- Propyne reacts with hypochlorous acid to give a major product as :-
Text Solution
|
- Which of the following is correct IUPAC name of: CH(3) - overset(CH(...
Text Solution
|
- The given reaction is:
Text Solution
|
- Alkaline hydrolysis of coconut oil gives:
Text Solution
|
- Which of the following pair shows positive value of: E(M^(+3)//M^(+2...
Text Solution
|
- Oxidation numbers of Cl-atoms in CaOCl2 (bleaching powder) :
Text Solution
|
- AgCl is crystallized from molten AgCl containing a little CdCl(2). The...
Text Solution
|
- If Ea for the forward and backward reaction is 150 and 260 kJ "mol"^(-...
Text Solution
|
- In thermodynamics, a process is called reversible when :
Text Solution
|
- Which of the following ligands have nitrogen as their donor atom ? e...
Text Solution
|
- In which of the following species bond angle decreases when all CI are...
Text Solution
|
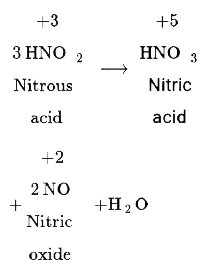
- For the following reaction, find the sum of the oxidation state of nit...
Text Solution
|
- Amongst the following, the total number of compounds which can be prep...
Text Solution
|
- 3-Methylbutan-2- ol - HI overset(Delta) to X. Identify the position...
Text Solution
|
- Benzene can be produced from hexane in the reversible reaction: C(6...
Text Solution
|
- A 2g sample containing KI and NaCl yielded 0.425g metallic palladium. ...
Text Solution
|
- If alcoholic KOH is added to 15. 7 g of 1 -chloropropane, then calcula...
Text Solution
|
- 0.20 mol of He and 1.00 mol of an unknown compound (vapor pressure 0.7...
Text Solution
|
- A hydrogen-like species can emit a maximum energy photon of 204 eV. It...
Text Solution
|