A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA TPC JEE MAIN TEST 40-PHYSICS
- Materials suitable for permanent magnet, must have which of the follo...
Text Solution
|
- Two particle of mass m each are fixed to a massless rod of length 2l ....
Text Solution
|
- The dimensional formula for magnetic flux is
Text Solution
|
- Ther is a telescope of magnifying power 5 and tube length 60 cm. What ...
Text Solution
|
- Find out Co-ordinats of centre of mass of uniform L shaped plate shown...
Text Solution
|
- An engine has an efficiency of 1/6. When the temperature of the sink i...
Text Solution
|
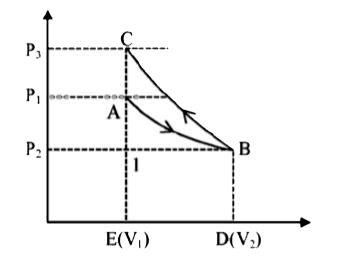
- An ideal gas expands isothermally from volume V(1) to V(2) and is then...
Text Solution
|
- P-V plots for two gases during adiabatic processes are shown in the fi...
Text Solution
|
- Suppose the distance between the slits in a double-slit experiment is ...
Text Solution
|
- Statemetn 1: The linear velocity of the individual smooth particles un...
Text Solution
|
- Two resistors 600Omega each are connected in series with a battery 8V....
Text Solution
|
- A dielectric of dielectric constant 3 fill up three fourths of the spa...
Text Solution
|
- A tap has water drops dripping from it at a steady rate of 3 drops per...
Text Solution
|
- One end of a spring is fixed at point O of a smooth fixed horizontal c...
Text Solution
|
- In an experiment for measuring sped of soung (v) in air by resonance t...
Text Solution
|
- Consider a horizontal water pipe connected to a pressure meter. When t...
Text Solution
|
- Consider a narrow glass U tube with the inner of the limbs being 2 mm ...
Text Solution
|
- A rod of height h is palced in a beaker of same radius. The height of ...
Text Solution
|
- Let M be the mass and L be the length of a thin uniform rod. In first ...
Text Solution
|
- In a certain thermodynamic process, pressure is a parabolic function o...
Text Solution
|