Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
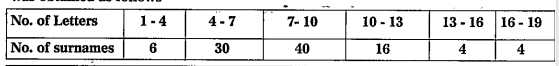
- 100 surnames were randomly picked up from a local telephone directory ...
Text Solution
|
- 100 surnames were randomly picked up from a local telephone directory...
Text Solution
|
- एक स्थाननीय टेलीफोन निर्देशिका से 100 कुलनाम लिए और उनमे प्रयुक्त अंग्...
Text Solution
|
- 100 surnames were randomly picked up from a local telephone directory ...
Text Solution
|
- 100 surnames were randomly picked up from a local telephone directory ...
Text Solution
|
- 100 surnames were randomly randomly picked up from a local telephone d...
Text Solution
|
- 100 surnames ware randomly picked up from a local telephone directory ...
Text Solution
|
- Find the mode of the given data 8,5,4,6,7,4,4,3,5,4,5,4,4,4,3
Text Solution
|
- 100 surnames were randomly picked up from a local telephone directory ...
Text Solution
|