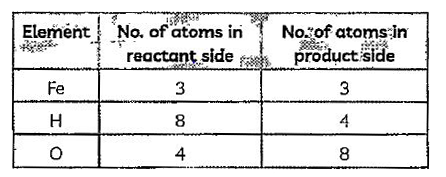
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following correctly represents a balanced chemical equati...
Text Solution
|
- Which of the following equation is correctly balanced ?
Text Solution
|
- Which one among the following equations is correctly balanced ?
Text Solution
|
- निम्नलिखित समीकरणों में से कौन-सी एक सही संतुलित है ?
Text Solution
|
- रासायनिक समीकरणों को निम्नलिखित में से किस नियम के सन्तुलित किया जाता ...
Text Solution
|
- Which of the following equations is (are) not correctly balanced ?
Text Solution
|
- Which of the following is not a balanced chemical equation?
Text Solution
|
- Which of the following information is not conveyed by a balanced chemi...
Text Solution
|
- Balance the following chemical equations.
Text Solution
|