A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROLYSIS
ICSE|Exercise Assertion and Reason Based Questions|4 VideosELECTROLYSIS
ICSE|Exercise Reason Based Questions|6 VideosCHEMISTRY-2015
ICSE|Exercise SECTION-II (40 Marks) Attempt any four questions from this Section.|49 VideosMETALLURGY
ICSE|Exercise UNIT TEST PAPER 6 - Metallurgy (Select the correct answer from the list A,B,C & given in each statement.) |5 Videos
Similar Questions
Explore conceptually related problems
ICSE-ELECTROLYSIS-Figure Based Questions
- Study the given figure and answer the question that follow : Nam...
Text Solution
|
- Study the given figure and answer the question that follow: Write...
Text Solution
|
- Study the given figure and answer the question that follow: Giv...
Text Solution
|
- Study the given figure and answer the question that follow: Whi...
Text Solution
|
- Study the given figure and answer the question that follow: Why...
Text Solution
|
- Study the given figure and answer the question that follow: Nam...
Text Solution
|
- Study the given figure and answer the question that follow: Nam...
Text Solution
|
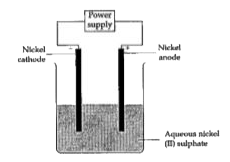
- An aqueous solution of nickel (II) sulphate was electrolysed using nic...
Text Solution
|