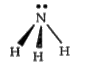A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SPECIMEN QUESTION PAPER (CHEMISTRY)-Questions
- The compound which is a non-electrolyte.
Text Solution
|
- Twice the vapour density gives
Text Solution
|
- The number of lone pairs of electrons in the nitrogen atom in ammonia ...
Text Solution
|
- Elements with similar valence shell configuration in a Periodic Table ...
Text Solution
|
- The gas liberated when Sodium Sulphite reacts with dilute sulphuric ac...
Text Solution
|
- Thickness of metal coating during electroplating depends on:
Text Solution
|
- Ionic bonding is seen in
Text Solution
|
- The molecular formula of an organic compound is C6H12O6 and the empiri...
Text Solution
|
- When an electron is added in the valence shell
Text Solution
|
- The most electronegative element is
Text Solution
|
- The bond in Carbon Tetrachloride is
Text Solution
|
- The type of bonding present in the nitrogen molecule
Text Solution
|
- A compound with empirical formula XY2 has vapour density equal to its ...
Text Solution
|
- Identify one statement that does not hold true for electrorefining of ...
Text Solution
|
- Write your observations for the chemical reactions and name the produc...
Text Solution
|
- The colour of the precipitate formed when ferrous ions react with ammo...
Text Solution
|
- During ionisation metals lose electrons, this change can be called :
Text Solution
|
- Give the reaction of oxide of metal that reacts with acid and alkali t...
Text Solution
|
- Which property decreases from left to right across the periodic table ...
Text Solution
|
- On the basis of electronic configuration the period and group of B(5)^...
Text Solution
|