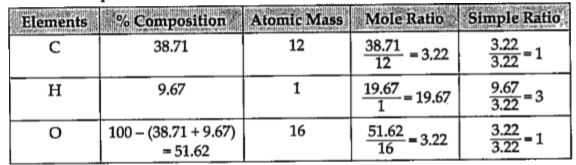
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 3 -MULTIPLE CHOICE QUESTION
- An element having atomic number 17 and belongs to halogens is
Text Solution
|
- How many valence electrons are present in Mg?
Text Solution
|
- Write the name of a non-metal of group 15.
Text Solution
|
- When fused lead bromide is electrolysed we observe:
Text Solution
|
- Arrange the following as per the instructions given in the brackets: ...
Text Solution
|
- A chloride which forms a precipitate that is soluble in excess of ammo...
Text Solution
|
- Sodium carbonate is a basic salt because it is a salt of a:
Text Solution
|
- A polar covalent bond will be formed in which one of these pair of ato...
Text Solution
|
- Sodium carbonate is a basic salt because it is a salt of a:
Text Solution
|
- The vessel in which electrolysis of lead bromide is carried out is:
Text Solution
|
- Arrange the following as per instructions given in the brackets: Cl,...
Text Solution
|
- A solution of the compound which gives a dirty green precipitate with ...
Text Solution
|
- Alkalis are :
Text Solution
|
- Aluminium has a tendency to loose ......... electrons.
Text Solution
|
- An organic compound contains carbon , hydrogen and oxygen . Its elemen...
Text Solution
|
- Which soln. becomes a deep/inky blue colour when excess of ammonium hy...
Text Solution
|
- The diagram given below is a part of Periodic Table. Study the table a...
Text Solution
|
- The diagram given below is a part of Periodic Table. Study the table a...
Text Solution
|
- The diagram given below is a part of Periodic Table. Study the table a...
Text Solution
|
- The diagram given below is a part of Periodic Table. Study the table a...
Text Solution
|