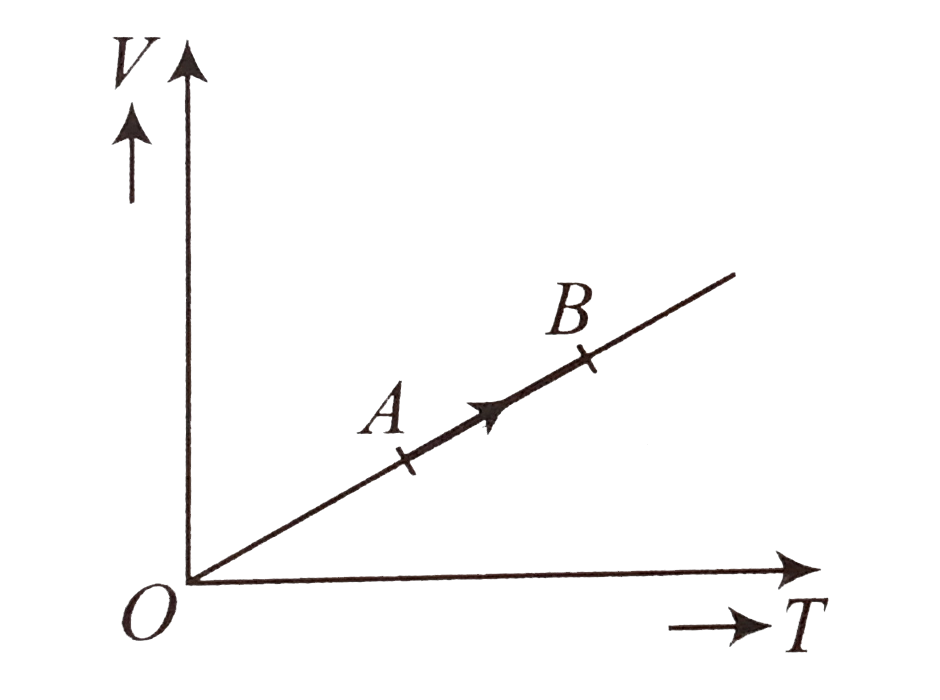
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The volume (V) of a monatomic gas varies with its temperature (T) as s...
Text Solution
|
- An ideal gas undergoes an isobaric process. If its heat capacity is C,...
Text Solution
|
- Volume versus temperature graph of two moles of helium gas is as shown...
Text Solution
|
- The volume (V) of a manatomic gas varies with its temperature (T) , as...
Text Solution
|
- The volume (V) of a monatomic gas varies with its temperature (T) as s...
Text Solution
|
- One mole of an ideal monatomic gas undergoes a linear process from A t...
Text Solution
|
- किसी एकपरमाणुक गैस के आयतन (V) में ताप (T) के साथ विचरण ग्राफ में दर्श...
Text Solution
|
- किसी गैस के लिए दाब P. आयतन V. तथा ताप T. आपस में p = (AT - BT^2)/v द्...
Text Solution
|
- 2 ग्राम हीलियम गैस के लिए आयतन (V) ताप (T) वक्र चित्रानुसार है। प्रक्र...
Text Solution
|