Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
MS CHOUHAN|Exercise LEVEL - 2 (SUBJECTIVE PROBLEMS )|13 VideosGENERAL ORGANIC CHEMISTRY
MS CHOUHAN|Exercise LEVEL - 2 (SUBJECTIVE PROBLEMS )|13 VideosCARBOXYLIC ACID AND THEIR DERIVATIVES
MS CHOUHAN|Exercise LEVEL-2( SUBJECTIVE PROBLEMS)|1 VideosGRIGNARD REAGENT
MS CHOUHAN|Exercise Level-2 (Subjective Problems)|6 Videos
Similar Questions
Explore conceptually related problems
MS CHOUHAN-GENERAL ORGANIC CHEMISTRY -LEVEL - 2
- Which of the compounds is the strongest Lewis base ?
Text Solution
|
- Rank the non-bonding electrons indicated by the arrows in order of inc...
Text Solution
|
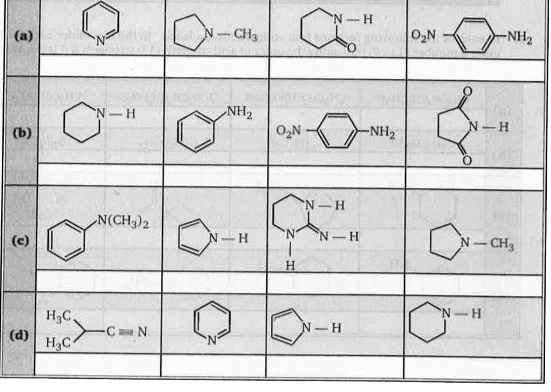
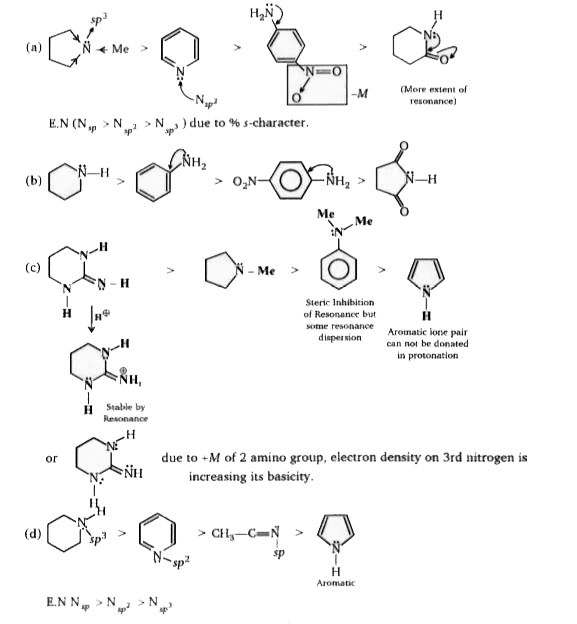
- In each of the following sections four nitrogen containing compounds a...
Text Solution
|
- For the two sets of acids shown below, rank their acidity most acidic ...
Text Solution
|
- rank their acidity most acidic to least acidic.
Text Solution
|
- In each of the following sections four compounds are listed. In the bo...
Text Solution
|
- In the two questions below, you are asked to rank the relative strengt...
Text Solution
|
- In the two questions below, you are asked to rank the relative strengt...
Text Solution
|
- In each of the following sections four compounds are listed. (Decreasi...
Text Solution
|
- Rank in the order of increasing basic strength.
Text Solution
|
- Compare acidic strength of the following (Write your answer in box).
Text Solution
|
- Arrange the hydrogens in increasing order of their acidic strengths.
Text Solution
|
- The compounds whose structures are shown below, incorporate a variety ...
Text Solution
|
- The compounds whose structures are shown below, incorporate a variety ...
Text Solution
|
- The compounds whose structures are shown below, incorporate a variety ...
Text Solution
|
- The compounds whose structures are shown below, incorporate a variety ...
Text Solution
|
- The compounds whose structures are shown below, incorporate a variety ...
Text Solution
|
- The compounds whose structures are shown below, incorporate a variety ...
Text Solution
|
- The compounds whose structures are shown below, incorporate a variety ...
Text Solution
|
- The compounds whose structures are shown below, incorporate a variety ...
Text Solution
|