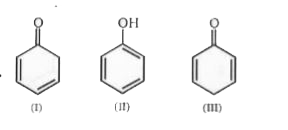
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM (STRUCTURAL & STEREOISOMERISM)
MS CHOUHAN|Exercise LEVEL 2|66 VideosISOMERISM (STRUCTURAL & STEREOISOMERISM)
MS CHOUHAN|Exercise LEVEL 2 (SUBJECTIVE PROBLEMS)|17 VideosHYDROCARBONS (ALKYNES)
MS CHOUHAN|Exercise Level -1|32 VideosIUPAC NAME
MS CHOUHAN|Exercise LEVEL-2 (SUBJECTIVE PROBLEMS ) |1 Videos
Similar Questions
Explore conceptually related problems
MS CHOUHAN-ISOMERISM (STRUCTURAL & STEREOISOMERISM)-LEVEL 2 (SUBJECTIVE PROBLEMS)
- The tautomer of II is :
Text Solution
|
- Number of chiral isomers are:
Text Solution
|
- Number of stereoisomer are
Text Solution
|
- Sum of number of stereoisomer (C) Degree of unsaturations in (D).
Text Solution
|
- How many 5 membered parent chain alkane are possible for C7H16?
Text Solution
|
- Theoretical possible geometrical isomer of
Text Solution
|
- The number of structural isomers possible for C5H11Br is
Text Solution
|
- Total number of plane of symmetry present in given compound is
Text Solution
|
- Total number of isomers for C4H6Br2 containing cyclobutane ring are ( ...
Text Solution
|
- Total number of structural isomers of C9H18 containing cyclohexane rin...
Text Solution
|
- How many structural isomers exist with the formula C(4)H(10)O?
Text Solution
|
- Write structures of different chain isomers of alkanes corresponding t...
Text Solution
|
- (a) to (x) ( Number of plane of symmetry) (b) to (y) (Number of me...
Text Solution
|
- Find out the total number of stereocentre in the given compound. CH3...
Text Solution
|
- Write the name of the organic compound which have the following struct...
Text Solution
|
- Find the total number of isomers of C7H14 (only 5-membered ring).
Text Solution
|
- x = number of compounds which undergoes Tautomerisation to form an Aro...
Text Solution
|
- If molecule is pyramidal, X stereoisomers are possible for : C(abcd)...
Text Solution
|