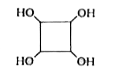
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MS CHOUHAN-GRIGNARD REAGENT-Level -2 (Comprehension)
- Grignard reagent is usually prepared by R-X+Mg overset(Et(2)O)rarr R...
Text Solution
|
- Grignard reagent is usually prepared by R-X+Mg overset(Et(2)O)rarr R...
Text Solution
|
- Grignard reagent is usually prepared by R-X+Mg overset(Et(2)O)rarr R...
Text Solution
|
- Grignard reagent is usually prepared by R-X+Mg overset(Et(2)O)rarr R...
Text Solution
|
- Grignard reagent is usually prepared by R-X+Mg overset(Et(2)O)rarr R...
Text Solution
|
- Grignard reagent is usually prepared by R-X+Mg overset(Et(2)O)rarr R...
Text Solution
|
- Grignard reagent is usually prepared by R-X+Mg overset(Et(2)O)rarr R...
Text Solution
|
- Match the column I and II. (Matrix)
Text Solution
|
- Match the column I and II. (Matrix) Match the missing reactant A...
Text Solution
|
- Match the column I and II. (Matrix)
Text Solution
|
- When 20 g of a compound (A) = M.F. (C(4)H(10)O(4)) reacts with exc...
Text Solution
|