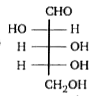
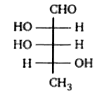
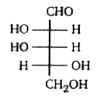
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MS CHOUHAN-BIOMOLECULES -LEVEL - 2
- Among the three compounds shown below, two yield the same product on r...
Text Solution
|
- Match the colume (I) and Column (II). (Matrix)
Text Solution
|
- One cyclic acetal form of D - galactose is shown above. Which atom ...
Text Solution
|
- In the presence of lactase, lactose breaks down into molecules of:
Text Solution
|
- Another name for Vitamin D is:
Text Solution
|
- Following diseases are caused by the deficiency of which vitamin: Poor...
Text Solution
|