Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MS CHOUHAN-ALCOHOL,ETHERS AND EPOXIDES-LEVEL-2
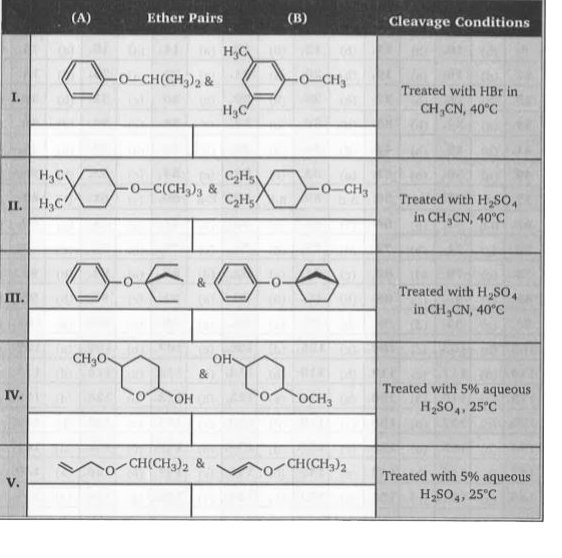
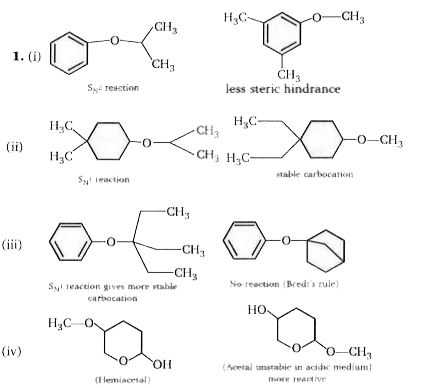
- Consider the pairs ofethers, numbered I throughV, shown below. To the ...
Text Solution
|
- Based on the above isomer answer the following (A to F). Which isome...
Text Solution
|
- Based on the above isomer answer the following (A to F). Which isome...
Text Solution
|
- Based on the above isomer answer the following (A to F). Which isome...
Text Solution
|
- Based on the above isomer answer the following (A to F). Which isome...
Text Solution
|
- Based on the above isomer answer the following (A to F). Which isome...
Text Solution
|
- Based on the above isomer answer the following (A to F). Which isome...
Text Solution
|
- Conversion (CH3 - CH3 rarr CH3 - CH2 - OH) " alkane " rarr alcohol is...
Text Solution
|
- Conversion R -CH2 - OH rarr R -CHO can be done by :
Text Solution
|
- Conversion R-CHO rarr R - CO2 H can be done by
Text Solution
|
- Coversion R - CO2H rarr R - CHO can be done by :
Text Solution
|
- Coversion R - CHO rarr R - CH2 -OH can be done by :
Text Solution
|
- Reduction R - CH2 - OH rarr R - CH3 can be done by :
Text Solution
|
- Which of the following is true for 3- methylbutanal ?
Text Solution
|
- This problem is an introduction to the planning of multistep syntheses...
Text Solution
|
- Which of the following is true for 3- methyl-2-butanone?
Text Solution
|
- Which of these methods would serve to prepare 1-phenyl-2-propanol?
Text Solution
|
- Match the Column (I) and (II) .
Text Solution
|
- Match the Column (I) and (II) .
Text Solution
|
- Ratio ofmoles of formaldehyde obtained in the reaction (1) and reactio...
Text Solution
|