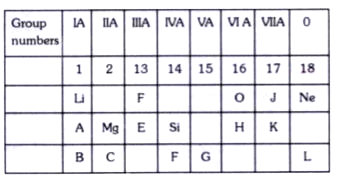
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ICSE-SAMPLE PAPER 04-QUESTIONS
- What is formed when zinc reacts with sodium hydroxide?
Text Solution
|
- Choose the correct answer from the options given below : Hydroxide o...
Text Solution
|
- The starting material which takes part in chemical reaction is called ...
Text Solution
|
- During ionisation metals lose electrons, this change can be called :
Text Solution
|
- Alkaline earth metals include:
Text Solution
|
- Methyl orange is :
Text Solution
|
- The formula which gives the simple ratio of each kind of atoms present...
Text Solution
|
- Why do atoms share electrons in covalent bonds?
Text Solution
|
- The hydroxide which is soluble in excess of NaOH is .............
Text Solution
|
- The metallic electrode which does not take part in an electrolytic rea...
Text Solution
|
- Identify the element belonging to third period and 17th group of the p...
Text Solution
|
- Brine is the common name of
Text Solution
|
- Covalent bond is formed between :
Text Solution
|
- Name the reagent from the following which can be used to distinguish z...
Text Solution
|
- The formula which gives the simple ratio of each kind of atoms present...
Text Solution
|
- Select the correct answer from the list given in brackets : When di...
Text Solution
|
- Consider the section of the periodic table given below. Note ...
Text Solution
|
- Consider the section of the periodic table given below. Note ...
Text Solution
|
- Consider the section of the periodic table given below. Note ...
Text Solution
|
- Consider the section of the periodic table given below : In this t...
Text Solution
|