A
B
C
D
Text Solution
Verified by Experts
X BOARDS-QUESTION PAPER 2022 TERM1-Section -B
- Which one of the following reactions is categorised as thermal decompo...
Text Solution
|
- Consider the pH value of the following acidic samples: The decre...
Text Solution
|
- Study the experimental set up shown in given figure and choose the cor...
Text Solution
|
- Which one of the following structures correctly depicts the compound C...
Text Solution
|
- The pair(s) which will show displacement reaction is/are (i) NaCl so...
Text Solution
|
- Which of the following salts do not have the water of crystalisation ?...
Text Solution
|
- Assertion (A) : Sodium hydrogen carbonate is used as an ingredient in ...
Text Solution
|
- Assertion (A) : Burning of Natural gas is an endothermic process. Re...
Text Solution
|
- Observe the diagram of an activity given below. What does it help to c...
Text Solution
|
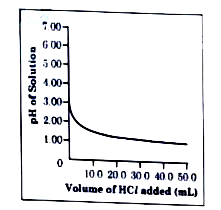
- 50.0 mL of tap water was taken in a beaker. Hydrochloric acid was adde...
Text Solution
|