A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -NSO QUESTION PAPER 2016 SET A-Achievers Section
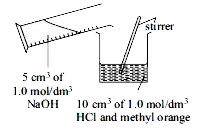
- In an experiment, 5 cm^3 of 1.0 mol/"dm"^3 NaOH solution is gradually ...
Text Solution
|
- The given figure shows the I-V curve (i) for a nichrome wire of fixed ...
Text Solution
|
- An organic compound 'A' on heating with concentrated H(2)SO(4) forms a...
Text Solution
|
- An organic compound 'A' on heating with concentrated H2 SO4 forms a c...
Text Solution
|