A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -CHANGES AROUND US-MULTIPLE CHOICE QUESTION
- Which of the following features are related to evaporation of water? ...
Text Solution
|
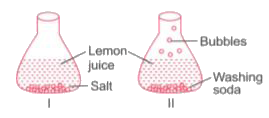
- Sudha took lemon juice in two flasks. She put salt in flask 1 and wash...
Text Solution
|
- Study the given Venn diagram carefully The centre point X rep...
Text Solution
|
- A metallic piece (X) is dropped into copper sulphate solution. After s...
Text Solution
|
- Read the given passage and fill in the blanks by selecting an appropri...
Text Solution
|
- Mark the incorrect statement
Text Solution
|
- Few changes are given below. Mark the correct option from the given co...
Text Solution
|
- Nitu mixed some iron filings with sulphur powder in a China dish. She ...
Text Solution
|
- What is common among the following phenomena? 1. Change of season...
Text Solution
|
- Observe the two figures given below. In figure la solution of sugar in...
Text Solution
|
- Identify the reversible and irreversible changes among the following: ...
Text Solution
|
- Study the given flow chart carefully,
Text Solution
|
- A potter shapes pots out of clay (Process 1). He then bakes the pots i...
Text Solution
|
- Match column with column Il and select the correct option from the giv...
Text Solution
|
- Consider the following phenomena : (i) Forest fire (ii) Growth of ...
Text Solution
|
- Observe the given figure carefully and select the correct statement.
Text Solution
|
- Swati classified a few changes occurring around us as shown in the tab...
Text Solution
|
- Read the given statements and mark the correct option Statement 1: ...
Text Solution
|
- Study the given Venn diagram and identify points 1, 2 and 3.
Text Solution
|
- Study the given Venn diagram. If 1 represents slaking of lime...
Text Solution
|