Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CBSE MODEL PAPER-SAMPLE PAPER 2022 TERM II-SECTION B
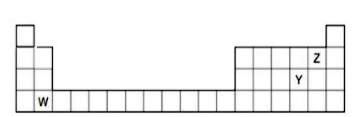
- The diagram below shows part of the periodic table. a. Which element...
Text Solution
|
- Choose an element from period 3 of modern periodic table that matches ...
Text Solution
|
- a. How many isomers are possible for the compound with the molecular f...
Text Solution
|
- A carbon compound ‘A’ having melting point 156K and boiling point 351K...
Text Solution
|
- Gas A, found in the upper layers of the atmosphere, is a deadly poison...
Text Solution
|