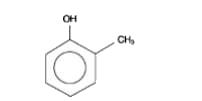
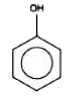
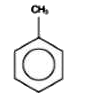
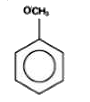
A
B
C
D
Text Solution
AI Generated Solution
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -OLYMPIAD - 2019 (CLASS 11)-Section B – Chemistry
- Eka-aluminium and Eka -silicon are known as :
Text Solution
|
- In which of the following molecule/ion all the bonds are not equal?
Text Solution
|
- With regard to the gaseous state of matter which of the following stat...
Text Solution
|
- Covalency of carbon in CO molecule is three because
Text Solution
|
- Enthalpy of sublimation of a substance is equal to :
Text Solution
|
- Ammonium carbonate when heated to 200^(@)C gives a mixture of NH(3) an...
Text Solution
|
- What will be the correct order of vapour pressure of water, acetone an...
Text Solution
|
- A standard hydrogen electrode has zero electrode potential because :
Text Solution
|
- The structure of an organic compound which on oxidation gives an acid ...
Text Solution
|
- Which one of the following statements regarding hydrogen peroxide is f...
Text Solution
|