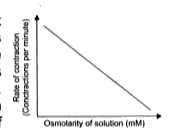
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -CELL - THE FUNDAMENTAL UNIT OF LIFE -MULTIPLE CHOICE QUESTIONS (ACHIEVERS SECTION (HOTS))
- Among the following cell organelles P, Q, R and S, which is the odd on...
Text Solution
|
- Four strips are cut from a fresh potato. The length of each strip is m...
Text Solution
|
- A unicellular protist X, which has contractile vacuole to remove exces...
Text Solution
|
- Refer to the given key and answer the following questions. (i) Membr...
Text Solution
|
- Refer to the given key and answer the following questions. (i) Membr...
Text Solution
|