Read the characteristics of three divisions of Animal Kingdom: Hemichordata, Echinodermata and Arthropoda and select the correct option.
1. Power of regeneration.
2. Excretory organ is proboscis gland.
3. Examples are Echinus, Holothuria.
4. Development includes tornaria larva.
5. Haemocoel present.
6. Examples are Limulus, Lepisma.
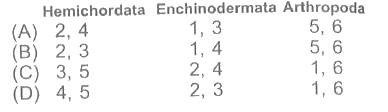
Read the characteristics of three divisions of Animal Kingdom: Hemichordata, Echinodermata and Arthropoda and select the correct option.
1. Power of regeneration.
2. Excretory organ is proboscis gland.
3. Examples are Echinus, Holothuria.
4. Development includes tornaria larva.
5. Haemocoel present.
6. Examples are Limulus, Lepisma.
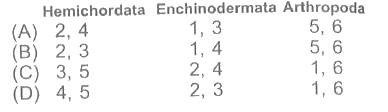
1. Power of regeneration.
2. Excretory organ is proboscis gland.
3. Examples are Echinus, Holothuria.
4. Development includes tornaria larva.
5. Haemocoel present.
6. Examples are Limulus, Lepisma.
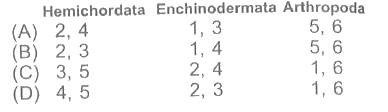
Text Solution
Verified by Experts
The correct Answer is:
A
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
Read the statements carefully and select the correct option for bacteria. 1. They are the sole memebers of the kingdom-monera. 2 . Cannot live in snow and deep oceans. 3 . No bacteria live in or on the other organisms as parasites. 4 . Have simple structure and complex behaviour.
Given below are the steps involved in oogenesis . Arrange them in proper sequence and select the correct option. 1. Formation of primary follicle due to development of granulosa cells. 2 Division of oogonia and formation of the primary oocyte. 3. Formation of follicles having antrum . 4. Formation of secondary follicles. 5. An unequal division leading to the formation of secondary oocyte i.e. ovum and the first polar body .
Study carefully the following stages of life cycle of malarial parasite i.e., Plasmodium. Arrange these stages in the correct sequence and select the correct answer. 1. Sporozoites leave the blood stream and enter the liver cells of man. 2. Sporozoites present in the salivary glands of female Anopheles mosquito are injected into the blood stream of man. 3. The parasite reproduces asexually in RRCs, resulting in bursting of RRCs and causing the cycles of fever, released parasites infect new RRCs. 4. The parasite reproduces asexually in liver cells, ultimately causing the rupturing of cells. 5. Two types of gametocytes i.e., microgametocytes and macrogametocytes develop in the RRCs. 6. Female Anopheles mosquito takes up the gametocytes with blood meal of an infected person. 7. Mature infective stage of the parasite i.e., sporozoites escape from intestine and migrate to the mosquito's salivary glands. 8. Fertilisation and developmental stages of the parasite take place in mosquito's stomach.
Read the passage given below and answer the following questions: Reduction of carboxylic acids and their derivatives plays an important role in organic synthesis, in both laboratory and industrial processes. Traditionally, the reduction is performed using stochiometric amounts of hydride reagents, generating stochiometric amounts of waste. A much more attractive, atom-economical approach is a catalytic reaction using H_2 , however, hydrogenation of carboxylic acid derivatives under mild conditions is a very challenging task, with amides presenting the highest challenge among all classes of carbonyl compounds. Very few examples of the important hydrogenation of amides to amines, in which the C-O bond is cleaved with the liberation of water (Scheme 1), were reported. C-O cleavage of amides can also be affected with silanes as reducing agents. We have now prepared the new, dearomatized, bipyridine-based pincer complex 3, catalyst 3(Here refered as Cat. 3). Remarkably, it efficiently catalyzes the selective hydrogenation of amides to form amines and alcohols (eq 1). The reaction proceeds under mild pressure and neutral conditions, with no additives being required. Since the reaction proceeds well under anhydrous conditions, hydrolytic cleavage of the amide is not involved in this process. (Balaraman, E., Gnanaprakasam, B., Shimon, L. J., & Milstein, D. (2010). Direct hydrogenation of amides to alcohols and amines under mild conditions. Journal of the American Chemical Society, 132(47), 16756-16758.) In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage. Assertion: N-methyl ethanamide on reaction with catalyst 3 will yield ethanol and methanamine. Reason: Use of Catalyst 3 brings about cleavage of C-N bond of amides
Read the passage given below and answer the following questions: Reduction of carboxylic acids and their derivatives plays an important role in organic synthesis, in both laboratory and industrial processes. Traditionally, the reduction is performed using stochiometric amounts of hydride reagents, generating stochiometric amounts of waste. A much more attractive, atom-economical approach is a catalytic reaction using H_2 , however, hydrogenation of carboxylic acid derivatives under mild conditions is a very challenging task, with amides presenting the highest challenge among all classes of carbonyl compounds. Very few examples of the important hydrogenation of amides to amines, in which the C-O bond is cleaved with the liberation of water (Scheme 1), were reported. C-O cleavage of amides can also be affected with silanes as reducing agents. We have now prepared the new, dearomatized, bipyridine-based pincer complex 3, catalyst 3(Here refered as Cat. 3). Remarkably, it efficiently catalyzes the selective hydrogenation of amides to form amines and alcohols (eq 1). The reaction proceeds under mild pressure and neutral conditions, with no additives being required. Since the reaction proceeds well under anhydrous conditions, hydrolytic cleavage of the amide is not involved in this process. (Balaraman, E., Gnanaprakasam, B., Shimon, L. J., & Milstein, D. (2010). Direct hydrogenation of amides to alcohols and amines under mild conditions. Journal of the American Chemical Society, 132(47), 16756-16758.) In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage. Assertion: The use of catalyst 3 is an efficient method of preparation of primary amines Reason: Use of catalyst 3 is a step down reaction.
Read the passage given below and answer the following questions: Reduction of carboxylic acids and their derivatives plays an important role in organic synthesis, in both laboratory and industrial processes. Traditionally, the reduction is performed using stochiometric amounts of hydride reagents, generating stochiometric amounts of waste. A much more attractive, atom-economical approach is a catalytic reaction using H_2 , however, hydrogenation of carboxylic acid derivatives under mild conditions is a very challenging task, with amides presenting the highest challenge among all classes of carbonyl compounds. Very few examples of the important hydrogenation of amides to amines, in which the C-O bond is cleaved with the liberation of water (Scheme 1), were reported. C-O cleavage of amides can also be affected with silanes as reducing agents. We have now prepared the new, dearomatized, bipyridine-based pincer complex 3, catalyst 3(Here refered as Cat. 3). Remarkably, it efficiently catalyzes the selective hydrogenation of amides to form amines and alcohols (eq 1). The reaction proceeds under mild pressure and neutral conditions, with no additives being required. Since the reaction proceeds well under anhydrous conditions, hydrolytic cleavage of the amide is not involved in this process. (Balaraman, E., Gnanaprakasam, B., Shimon, L. J., & Milstein, D. (2010). Direct hydrogenation of amides to alcohols and amines under mild conditions. Journal of the American Chemical Society, 132(47), 16756-16758.) In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage. Assertion: Aniline can be prepared from suitable amide using catalyst 3 Reason: The use of catalyst 3 is limited to aliphatic amides only.
Read the passage given below and answer the following questions: Reduction of carboxylic acids and their derivatives plays an important role in organic synthesis, in both laboratory and industrial processes. Traditionally, the reduction is performed using stochiometric amounts of hydride reagents, generating stochiometric amounts of waste. A much more attractive, atom-economical approach is a catalytic reaction using H_2 , however, hydrogenation of carboxylic acid derivatives under mild conditions is a very challenging task, with amides presenting the highest challenge among all classes of carbonyl compounds. Very few examples of the important hydrogenation of amides to amines, in which the C-O bond is cleaved with the liberation of water (Scheme 1), were reported. C-O cleavage of amides can also be affected with silanes as reducing agents. We have now prepared the new, dearomatized, bipyridine-based pincer complex 3, catalyst 3(Here refered as Cat. 3). Remarkably, it efficiently catalyzes the selective hydrogenation of amides to form amines and alcohols (eq 1). The reaction proceeds under mild pressure and neutral conditions, with no additives being required. Since the reaction proceeds well under anhydrous conditions, hydrolytic cleavage of the amide is not involved in this process. (Balaraman, E., Gnanaprakasam, B., Shimon, L. J., & Milstein, D. (2010). Direct hydrogenation of amides to alcohols and amines under mild conditions. Journal of the American Chemical Society, 132(47), 16756-16758.) In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage. Assertion: Use of hydride catalyst or hydrogen brings about cleavage of C-O bond in amides. Reason: Hydride catalyst or hydrogen cause to reduction of amides.
After receiving the lamp, Aladdin and Abu are on their way to get out from the cave when they found a statue of Sir Hussain, one of the most powerful Royal General to walk on Earth. Below the statue is written a real life problem that Sir Hussain solved and in order to get out of the cave one must solve this riddle. Aladdin out of options starts reading the riddle- Once upon a time in the Kingdom far far away lived Sir Hussain, the chief Royal General. He was very proud of his men and he liked to invite the King to come and watch drill exer- cises which demonstrated the fighting techniques and tactics of the squad he was in charge of. But time went by and one-day Sir Hussain had a major argument with an old witch (there were rumours that the argument occurred after the general spoke badly of the witch flying techniques. That seemed to hurt the old witch very deeply). As the result of the argument, the witch put a rather strange curse upon the general. It sounded all complicated and quite harmless: "If the squared distance between some two soldiers equals to 5, then those soldiers will conflict with each other!" The drill exercises are held on a rectangular n × m field, split into nm square 1 × 1 segments for each soldier. Thus, the square of the distance between the soldiers that stand on squares (x_(1), y_(1)) and (x_(1),y_(2)) equals exactly (x_(1)-x_(2))^(2)+(y_(1)-y_(2))^(2) . Now not all nm squad soldiers can partici- pate in the drill exercises as it was before the old witch curse. Unless, of course, the general wants the soldiers to fight with each other or even worse. For example, if he puts a soldier in the square (2, 2), then he cannot put soldiers in the squares (1, 4), (3, 4), (4, 1) and (4, 3) — each of them will conflict with the soldier in the square (2, 2). Find the sum of maximum number of soldiers that can be simultaneously positioned on this field for each of the following cases i) 1002 2 ii) 451 2 iii) 780 2 iv) 121 2
After receiving the lamp, Aladdin and Abu are on their way to get out from the cave when they found a statue of Sir Hussain, one of the most powerful Royal General to walk on Earth. Below the statue is written a real life problem that Sir Hussain solved and in order to get out of the cave one must solve this riddle. Aladdin out of options starts reading the riddle- Once upon a time in the Kingdom far far away lived Sir Hussain, the chief Royal General. He was very proud of his men and he liked to invite the King to come and watch drill exer- cises which demonstrated the fighting techniques and tactics of the squad he was in charge of. But time went by and one-day Sir Hussain had a major argument with an old witch (there were rumours that the argument occurred after the general spoke badly of the witch flying techniques. That seemed to hurt the old witch very deeply). As the result of the argument, the witch put a rather strange curse upon the general. It sounded all complicated and quite harmless: "If the squared distance between some two soldiers equals to 5, then those soldiers will conflict with each other!" The drill exercises are held on a rectangular n × m field, split into nm square 1 × 1 segments for each soldier. Thus, the square of the distance between the soldiers that stand on squares (x_(1), y_(1)) and (x_(1),y_(2)) equals exactly (x_(1)-x_(2))^(2)+(y_(1)-y_(2))^(2) . Now not all nm squad soldiers can partici- pate in the drill exercises as it was before the old witch curse. Unless, of course, the general wants the soldiers to fight with each other or even worse. For example, if he puts a soldier in the square (2, 2), then he cannot put soldiers in the squares (1, 4), (3, 4), (4, 1) and (4, 3) — each of them will conflict with the soldier in the square (2, 2). Find the sum of maximum number of soldiers that can be simultaneously positioned on this field for each of the following cases i) 53 xx 81 II) 2 xx 103 III) 1 xx 104
SCIENCE OLYMPIAD FOUNDATION -DIVERSITY IN LIVING ORGANISMS -ACHIEVERS SECTION (HOTS)
- Read the characteristics of three divisions of Animal Kingdom: Hemicho...
Text Solution
|
- Study the given flow chart. Which of the following are the correct exa...
Text Solution
|
- A list of animals (i-vii) is given. Classify them as acoelomates, coel...
Text Solution
|
- Refer to the given dichotomous key and answer the following questions....
Text Solution
|
- Refer to the given dichotomous key and answer the following questions.
Text Solution
|
- Read the given passage. Pteridophytes are the first plants with X t...
Text Solution
|