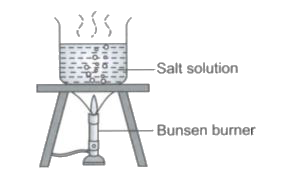
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -IS MATTER AROUND US PURE -MULTIPLE CHOICE QUESTION
- A few common substances are listed below. (i) Air, (ii) Graphite, (iii...
Text Solution
|
- Nidhi took two China dishes and marked them as I and II. In C...
Text Solution
|
- Which of the following statements is/are correct ? 1. Centrifugation m...
Text Solution
|
- Boiling points of a few gases are given below: If liquid mixt...
Text Solution
|
- Soham, a class 9 student mixed some iron filings and sulphur in a Chin...
Text Solution
|
- Sheela heated a mixture of iodine and common salt by keeping an invert...
Text Solution
|
- Geetika poured 20 g of salt into 200 mL of water in a beaker. She stir...
Text Solution
|
- A food sample was tested for the presence of components P, Q, R and S ...
Text Solution
|
- The constituents of a heterogeneous mixture are X, Y and Z. If mixture...
Text Solution
|
- A few mixtures are given in the box.
Text Solution
|
- What is the function of X in the given experimental set-up?
Text Solution
|
- Which of the following solutions has the highest mass by mass percenta...
Text Solution
|
- Select the correct statement(s). I. A solution in which size of th...
Text Solution
|
- Which of the following groups of students employed the correct method ...
Text Solution
|
- Read the given passage and fill in the blanks by selecting an appropri...
Text Solution
|
- Study the given Venn diagram carefully.
Text Solution
|
- Match column with column II and select the correct option from the giv...
Text Solution
|
- Study the given flow chart and select the correct statements. ...
Text Solution
|
- Siddharth dissolved a mixture into water and then filtered it. Solid X...
Text Solution
|
- Which of the following statements about the given experimental set-up ...
Text Solution
|