A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -ATOMS AND MOLECULES -ACHIEVERS SECTION (HOTS)
- Two samples X and Y of a pure substance obtained by two different meth...
Text Solution
|
- Which of the following represents the correct number of moles of each ...
Text Solution
|
- Which of the following molecules has highest number of particles?
Text Solution
|
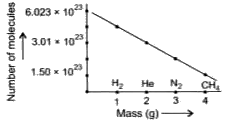
- The graphical representation of number of molecules of different gases...
Text Solution
|
- Sulphate of a divalent metal M exists in hydrated form. If 0.10 mol of...
Text Solution
|