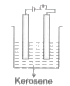
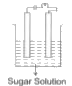
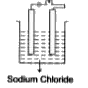A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -CHEMICAL EFFECTS OF ELECTRIC CURRENT-ACHIEVERS SECTION (HOTS)
- In which of the following will the bulb glow?
Text Solution
|
- There are two different solutions in set up P and Q as shown in figure...
Text Solution
|
- Four substances were tested for their electrical conductivity by placi...
Text Solution
|
- Four substances were tested for their electrical conductivity by placi...
Text Solution
|
- The bumpers and door handles of motor cars, taps etc. are coated with...
Text Solution
|
- Which of the following compound(s) is manufactured by using the chemic...
Text Solution
|