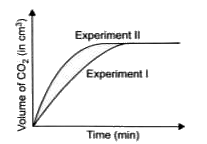
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -CHEMICAL REACTIONS AND EQUATIONS-MULTIPLE CHOICE QUESTIONS
- Study the given experimental set-up. If in test tubes I and III, blac...
Text Solution
|
- A small amount of a light green coloured compound X is heated in a tes...
Text Solution
|
- A small amount of calcium oxide (quick lime) is taken in a beaker. Wat...
Text Solution
|
- Observe the given reaction carefully and identify (i), (ii), (iii) and...
Text Solution
|
- Select the incorrect match(es). (i) Burning of magnesium ribbon - Comb...
Text Solution
|
- A student wrote three statements about rancidity : I. When fats and oi...
Text Solution
|
- Metal X is found in earth's crust. This metal forms a reddish brown su...
Text Solution
|
- Two colourless solutions X and Y were mixed together. On mixing, a yel...
Text Solution
|
- x, y and z in the given reaction are respectively
Text Solution
|
- 5 mL of sodium sulphate solution is taken in a test tube and 5 mL of b...
Text Solution
|
- Study the given reaction and fill in the blanks by selecting an approp...
Text Solution
|
- Which of the following does not represent a redox reaction ?
Text Solution
|
- Four test tubes were taken and marked 1, 2, 3 and 4 respectively. 2 mL...
Text Solution
|
- Read the following statements carefully and state (T) for true and (F)...
Text Solution
|
- Read the given statements and mark the correct option. Statement 1 : W...
Text Solution
|
- Which of the following reactions are exothermic in nature ? (i) Evapo...
Text Solution
|
- Marble chips or calcium carbonate react with hydrochloric acid as: Ca...
Text Solution
|
- Four students P, Q, R and S noted the initial colour of the solutions ...
Text Solution
|
- Observe the given figure carefully. Which of the following observation...
Text Solution
|
- Rupali, a class 10 student has set up the apparatus as shown in the fi...
Text Solution
|