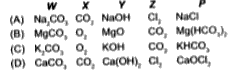A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -ACIDS, BASES AND SALTS-ACHIEVERS SECTION (HOTS)
- A metal carbonate W on reacting with an acid gives a gas X which when...
Text Solution
|
- Ayaan tested the nature of a few common substances with phenolphthale...
Text Solution
|
- While demonstrating acid-base reactions, Ms. Prabha, a science teache...
Text Solution
|
- Aqueous solutions of salts are either acidic, basic or neutral. A few...
Text Solution
|
- Observe the given figure carefully, which represents the decomposition...
Text Solution
|
- Study the given flow chart carefully and fill in the blanks by choosi...
Text Solution
|