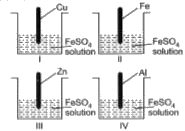
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -METALS AND NON-METALS-ACHIEVERS SECTION (HOTS)
- In which of the following beakers reaction will take place?
Text Solution
|
- Ritika took four metals P, Q, R and S and added them to following solu...
Text Solution
|
- Observe the given figure carefully. The residue left behind in the cr...
Text Solution
|
- Read the given statements carefully. I. Metals are generally ductile a...
Text Solution
|
- Read the given passage carefully and fill in the blanks by selecting ...
Text Solution
|
- Read the given statements carefully and state (T) for true and (F) fo...
Text Solution
|