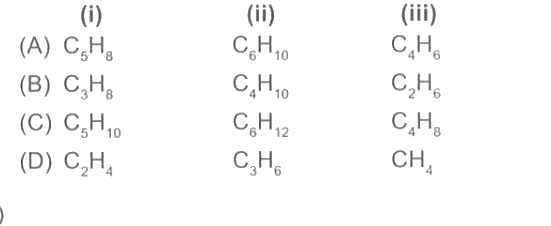
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -CARBON AND ITS COMPOUNDS-ACHIEVERS SECTION (HOTS)
- IUPAC names of a few esters are given below: 1. Ethyl propanoate ...
Text Solution
|
- Rohan was given three unknown organic compounds P, Q and R. To identif...
Text Solution
|
- For the same number of carbon atoms, three hydrocarbons P, Q and R ha...
Text Solution
|
- Which of the following represents the correct increasing order of vol...
Text Solution
|
- Read the given passage and fill in the blanks by choosing an appropria...
Text Solution
|