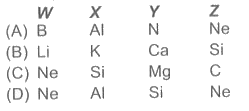A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SCIENCE OLYMPIAD FOUNDATION -PERIODIC CLASSIFICATION OF ELEMENTS-ACHIEVERS SECTION (HOTS)
- Electronic configurations of elements P, Q, R and S are given below : ...
Text Solution
|
- A brief information about elements W, X, Y and Z is given below : W : ...
Text Solution
|
- The given part of the periodic table represents period 3 containing el...
Text Solution
|
- A brief information about the element X is given below : The formu...
Text Solution
|
- The positions of four elements K, L, M and N in the periodic table are...
Text Solution
|